- oxygen has two forms O2 and O3
- allotropes different forms of the same element in the same state
- 21 percent dry air is O2
- oxygen attracts electrons from other elements
- oxygen with other metals from oxides
- peroxide O22- , superoxide O2-
- compounds with these ions react with themselves to produce an oxide and O2
- sulfur several allotropic forms
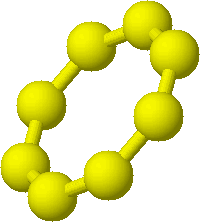 most common and most stable S8, usually written as S(s)
most common and most stable S8, usually written as S(s)- sulfur and its compounds burned in oxygen to form sulfur dioxide