Chapter 8: Basic Concepts of Chemical Bonding
- ionic bond electrostatic forces between ions of opposite charges
- transfers electrons
- metals and nonmetals
- covalent bond sharing of electrons
- nonmetallic elements
- metallic bonding bonding between metal atoms
8.1 Lewis Symbols and the Octet Rule
- valence electrons involved in chemical bonding
- electron-dot symbol shows valence electrons of atoms at bonds with other atoms
- octet rule atoms tend to lose or gain electrons until they have eight valence electrons
8.2 Ionic Bonding
- metal of low ionization energy and nonmetal with high affinity
- exothermic
8.2.1 Energetics of Ionic Bond Formation
- ionic compounds stable because of attraction between opposite charges of ions
- lattice energy energy required to separate one mole of a solid ionic compound into its gaseous ions
- ionic compounds are hard, brittle, high melting points
- potential energy of two interacting charged particles:
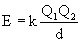 (Q1 and Q2 = charges of particles, d = distance between centers, k = constant = 8.99 x 109 J-m/C2
(Q1 and Q2 = charges of particles, d = distance between centers, k = constant = 8.99 x 109 J-m/C2- attractive interaction increases as magnitudes of charges increase as distance between centers decreases
- lattice energy increases as charges on ions increase and as radii decrease
- magnitude of lattice energy depends on ionic charges
8.2.2 Electron Configuration of Ions of the Representative Elements
- never find ionic compounds that contain Na2+ and others like it
- increase in lattice energy not enough to compensate for second ionization energy
- addition of electrons to a higher shell is energetically unfavorable
8.2.3 Transition-Metal Ions
- in forming ions, transition metals lose valence-shell s electrons first, then as many d electrons as are required to reach the charge of the ion
8.2.4 Polyatomic Ions
- a polytomic ion acts as one charged specie in forming ionic compounds
8.3 Sizes of Ions
- size of ion depends on nuclear charge, number of electrons, outer-shell orbitals
- cations are smaller than parent atoms
- anions larger than parent atom
- ion of same charge, size increases down a group
- as principal quantum number increases, size of ion and parent atom increases
- isoelectronic series ions having the same number of electrons
- increase in nuclear charge, decrease in atomic radius
8.4 Covalent Bonding
- lewis structures: shared electrons shown as lines and unshared as dots
8.4.1 Multiple Bonds
- distance between bonded atoms decreases with increasing shared electron pairs
8.5 Bond Polarity and Electronegativity
- bond polarity describes sharing of electrons
- nonpolar covalent bond electrons shared equally between atoms
- polar covalent bond one atoms has greater attraction toward for bonding electrons
- if large ionic bond occurs
8.5.2 Electronegativity
- electronegativity ability of an atom in a molecule to attract electrons to itself
- Linus Pauling first person to develop electronegativity scale
- Electronegativity increases across period
- Decreases down group
8.5.3 Electronegativity and Bond Polarity
- use difference of electronegativity to determine polarity
- greater difference of electronegativity more polar the bond
8.6 Drawing Lewis Structures
- 1) sum the valence electrons from all atoms
- 2) write symbols for atoms and connect with single bond
- 3) complet octets of atoms bonded to central atom
- place leftover electrons on central atom even if more than an octet
- if not enough for octet use double bond or triple bond
8.6.1 Formal Charge
- formal charge charge an atom in a molecule would have if all atoms had same electronegativity
- calculate formal charge:
- 1) all of unshared electrons assigned to original atom
- 2) half bonding electrons assigned to each atom in bond
- formal charge of atom equal number of valence electrons in isolated atom, minus number of electrons assigned to atom in Lewis structure
8.7 Resonance Structures
- alternate Lewis structures of same molecule
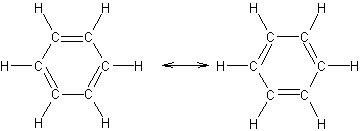 8.7.1 Resonance in Benzene
8.7.1 Resonance in Benzene
- lewis structures of benzene
8.8 Exceptions of the Octet Rule
- 1) molecules with odd number of electrons
- 2) molecules in which an atom has less than an octet
- 3) molecules in which an atom has more than on octet
8.8.1 Odd Number of Electrons
- ClO2, NO, NO2
- Octet cannot be achieved
8.8.2 Less than an Octet
- compounds of boron and beryllium
8.8.3 More than an Octet
- larger cental atom, the larger the number of atoms that can surround it
- expanded valence shells occur when central atom bonded to smallest most electronegative atoms
8.9 Strengths of Covalent Bonds
- bond enthalpy enthalpy change for breaking a particular bond in a mole of gaseous substance
- bond enthalpy always positive
8.9.1 Bond Enthalpies and the Enthalpies of Reactions
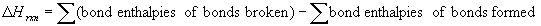
- bond enthalpies derived for gaseous molecules and are averaged values
8.9.2 Bond enthalpy and Bond Length
- bond length distance between the nuclei of bonded atoms
- as number bonds increases, the bond grows shorter
8.10 Oxidation Numbers
- 1) oxidation number of element in elemental from is zero
- 2) oxidation number of monoatomic ion is same as charge
- 3) in binary compounds element with greater electronegativity assigned a negative oxidation umber equal to charge in simple ionic compound of element
- 4) sum of the oxidation numbers equal zero for electrically neutral compound and equals overall charge for ionic species
8.10.1 Oxidation Numbers and Nomenclature
- naming binary compounds: one for ionic compounds and other for molecular compounds
- less electronegative element is given first, then more electronegative atom with ide ending
- compounds of metals in higher oxidation states molecular rather than ionic
|
Ionic |
Molecular |
|
MgH2 magnesium hydride |
H2S dihydrogen sulfide |
|
FeF2 iron(II) fluoride |
OF2 oxygen difluoride |
|
Mn2O3 manganese(III) oxide |
Cl2O3 dichlorine trioxide |
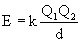 (Q1 and Q2 = charges of particles, d = distance between centers, k = constant = 8.99 x 109 J-m/C2
(Q1 and Q2 = charges of particles, d = distance between centers, k = constant = 8.99 x 109 J-m/C2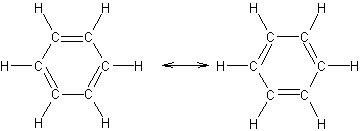 8.7.1 Resonance in Benzene
8.7.1 Resonance in Benzene