

Project Leader
Postdoctoral Researcher
yancy_riddle@yahoo.com
Diploma student
(dr.ing. student starting 6/2002)
hakoh@stud.ntnu.no
Institute Leader
nils.ryum@chembio.ntnu.no
 |
 |
|
| Yancy W.
Riddle, Ph.D.
Project Leader Postdoctoral Researcher yancy_riddle@yahoo.com |
Håkon
Hallem
Diploma student (dr.ing. student starting 6/2002) hakoh@stud.ntnu.no |
Nils Ryum,
prof. dr. techn.
Institute Leader nils.ryum@chembio.ntnu.no |
| Location:
Institute for Materials Technology and Electrochemistry Norwegian University of Science and Technology (NTNU) Alfred Getzvei 2b N-7491 Trondheim, Norway |
Relevant references &
resources:
Sc related resources |
Research Area: Study of Recrystallization in Aluminium Alloys
Current Research Topic: Addition
of Sc and Zr to Aluminium Alloys: Improving Recrystallization Resistance
Introduction:
Preventing recrystallization
in wrought aluminium alloys may result in the retention of strength, hardness,
corrosion resistance, fracture toughness, as well as decrease weld cracking.
These properties and more are often vital for the success of an aluminum
alloy in a given application. For the same reasons the alloy may
be required to survive long elevated temperature conditions in-service
and retain an unrecrystallized state. However careful control of
alloy composition and material processing conditions are needed if
these conditions are to be met. In particular the various processing
temperatures, processing times, and severity of deformation all contribute
to the challenge of recrystallization prevention.
In this research we employ various dispersoid phases to pin grain and subgrain boundaries thus preventing recrystallization. This is termed "Zener drag". The effectiveness of dispersoids varys with composition, size, volume fraction, distribution, and degree of coherency to the matrix. To date Al(3)Zr is the most effective dispersoids in use in aluminium alloys. Unfortunately Al(3)Zr is often difficult to precipitate resulting in strongly heterogeneous distributions having a less than optimal ability to retard recrystallization.
Recent research has shown scandium, when precipitated as Al(3)Sc, to be a particularly effective Zener drag agent. Ease of nucleation, high volume fraction, and homogeneous distribution contribute to its effectiveness. Furthermore scandium heat treatment is compatible with current practices making it an attractive addition to aluminium. However Al(3)Sc coarsens much faster than Al(3)Zr reducing its usefulness over longer high temperature annealing times. It has been discovered that combining Zr and Sc in the same alloy can, with proper heat treatment, result in an Al(3)(Sc, Zr) dispersoid which takes advantage of the positive properties of Al(3)Zr and Al(3)Sc. Al(3)(Sc, Zr) nucleates rapidly and homogeneously with a high volume fraction and slow coarsening rate (compared to Al(3)Sc). The reader is referred to (M.Sc.) and (Ph.D.) for more detailed information regarding the behavior of Al-Sc-Zr alloys. There is currently very limited use of scandium in industry making it a very expensive alloying addition. Further understanding of its potency, discovery of alternate sources, and increased demand for use in aluminium alloys may significantly drop scandium's price in the future.
Our goals:
Prevention of
recrystallization in some aluminum alloys is increased above the current
limit by adding both Sc
and Zr. Neither element acting alone
can provide this level of recrystallization resistance. Relatively
little is known about Al3(Sc,Zr). Our goal is to continue characterizing
this new Al3(Sc,Zr) dispersoid phase, understand why it is so effective,
and use this information for optimizing its performance. To
accomplish this we are utilizing optical, scanning, and transmission electron
microscopy, microprobe analysis, and a variety of mechanical tests.
The Al-Sc-Zr system may also provide a suitable alloy for studying very
early nucleation behavior which is of interest to a more general academic
metallurgical audience. For this we are planning an experiment using
the ESRF syncrotron.
Recent Results:
Recently we
have been improving the recrystallization properties of an experimental
extrudable Al-Mn-Mg alloy by adding Zr and Sc to form Al3(Sc, Zr) dispersoids.
Both commercial purity and high purity alloys are under investigation.
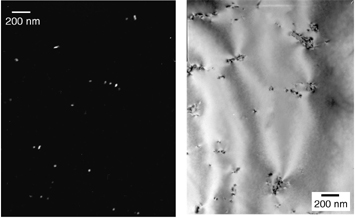
Without Sc addition we find the precipitation of Al(3)Zr following preheating
to be very poor and heterogeneous as demonstrated in figure 1a. No
alternate heat treatment was found to improve the distribution of Al(3)Zr.
With addition of Sc both the distribution and volume fraction of dipsersoids,
Al3(Sc,Zr) in this case, are both dramatically improved as seen in figure
1b. The micrographs in figure 1 are from a transmission electron
microscope (TEM).
 |
 |
| Figure 1a: Distribution of Al3Zr in an Al-Mn-Zr alloy | Figure 1b: Distribution of Al3(Sc,Zr) in an Al-Mn-Zr-Sc alloy |
After extrusion
these alloys were subject to annealing at 500, 550, or 600 C for 1 hour
and recrystallization behavior observed in the optical microscope using
anodized samples. The grain structures of the non-Sc and Sc alloy
shows markedly different behavior as demonstrated in figure 2. Without
Sc surface recrystallization begins after 1 hour at 500C with complete
recrystallization occuring after 1 hour at 550 C. By adding Sc to
the alloy recrystallization is surpressed to at least 600C for 1 hour with
only a thin layer of recrystallized material forming at the surface.
 |
| Figure 2: Optical micrographs of extruded material after 1hour
at 500, 550, and 600C
showing degree of recrystallization |
The details of the current research will be published at the 8th International Conference on Aluminum Alloys in Cambridge, England in July 2002. If you are interested in this research and/or would like more information please contact us. Check back for periodic updates.
Last update: November 13, 2001.