
Bio 201 LAB

9/20/00
Describe the difference between mean, range and standard deviation.
The mean is the sum off all the values in a set of values divided by the number of values in the set.
The range is the highest value and lowest value in a set of values.
Standard deviation is the square root of the variance.
![]()
| Table 4.1 | Calculated Water
Volume (ml) |
% Error (+ or -) | (Measured Vol -
Mean Vol) |
(Measured Vol -
Mean Vol)?#060;/td> | |||
| Serge | 38.60 | -3.50 | +0.60 | 0.36 | |||
| Kevin | 37.64 | -5.90 | -0.36 | 0.13 | |||
| Carmella | 34.12 | -14.70 | -3.88 | 15.05 | |||
| Todd | 38.70 | -3.25 | +0.70 | 0.49 | |||
| Whetkey | 43.50 | +8.75 | +5.50 | 30.20 | |||
| Rick | 37.40 | -6.50 | -0.60 | 0.36 | |||
| Craig | 37.71 | -5.73 | -0.29 | 0.08 | |||
| Joe | 37.15 | -7.13 | -0.85 | 0.72 | |||
| Ebon | 36.24 | -9.40 | -1.76 | 3.10 | |||
| Christine | 37.75 | -5.63 | -0.25 | 0.06 | |||
| Chesney | 40.01 | +0.03 | +2.01 | 4.04 | |||
| George | 37.94 | -5.15 | -0.06 | 0.00 | |||
| Elizabeth | 38.64 | -3.40 | +0.64 | 0.41 | |||
| Constance | 36.80 | -8.00 | -1.20 | 1.44 | |||
| Mike Casper | 36.99 | -7.53 | -1.01 | 1.02 | |||
| Jess | 38.79 | -3.03 | +0.79 | 0.62 | |||
Sum(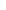 ) ) |
607.98 | Sum(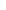 ) ) |
-80.07 | 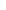 |
58.08 | ||
| Range | (34.12)-(43.50) | Range | (-14.70)-(+1.88) | 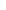 /(N-1) /(N-1) |
3.87 | ||
| Number of
observations (N) |
16 | Number of
observations (N) |
16 | ||||
Mean( /N) /N) |
38.00 | Mean( /N) /N) |
-5.00 |   /(N-1) /(N-1) |
1.97 |
![]()
![]()
Explain how standard curves can be used to determine the concentration
of a substance in solution.
Given a variable condition that is in a fixed ratio to the concentration if you plot known
concentrations you can determine unknown concentrations with that variable condition
since the variable condition is directly linked to the concentration any plotted
points for unknown concentrations should be in line with the known plotted
points on a standard curve graph.
![]()
| Table 4.3 | Concentration and absorbance for eight dye solutions at 600 nanometers. | |||
| Tube | ml of Dye | ml of H2O | Concentration | A |
| 1 | 0 | 8 | 0.0000 mg/ml | 0.00 |
| 2 | 1 | 7 | 0.1200 mg/ml | 0.20 |
| 3 | 2 | 6 | 0.0615 mg/ml | 0.39 |
| 4 | 3 | 5 | 0.0428 mg/ml | 0.56 |
| 5 | 4 | 4 | 0.0324 mg/ml | 0.74 |
| 6 | 5 | 3 | 0.0320 mg/ml | 0.75 |
| 7 | 6 | 2 | 0.0252 mg/ml | 0.95 |
| 8 | 7 | 1 | 0.0250 mg/ml | 0.96 |
| 9 | 8 | 0 | 0.0200 mg/ml | 1.20 |
| 10 | unknown | unknown | 0.0533 mg/ml | 0.45 |
![]()
![]()
9/27/00
![]()
In this lab we used chromatography to separate amino acids and
nucleotides. Discuss briefly how chromatography works,
and why it is an effective way to separate molecules.
Chromatography is when a solute is dragged up a stationary component by a moving component.
Smaller particles of the solute tend to move farther than larger particles of the solute.
The distance travelled is also affected by the solute's affinity either the stationary
component or the moving component. Different particles that the solute is
constituted by move independantly to one another according to their
size and affinity for either the stationary component or the moving
component.
![]()
In the lab we created a chromatograph separating nucleotides. Include either the
original, or photocopied version of this chromatograph, and determine
unknown nucleotides, by comparing it's position to the known
nucleotides.
Unknown A could very well be Unknown B but I would be willing to bet money that
neither could be Cytosine. Since both the unknowns had their bottoms
as low as Uracil and Cytosine but their tops as high as both Uracil and
Adenine I think our chromatograph is inconclusive.
![]()
We learned about three major major macromolecules today in lab, polysaccharides,
proteins, and nucleic acids.
For each macromolecule: (polysaccharides, proteins, nucleic acids)
a. Name the monomers which make up each macromolecule
b. Describe how we visualized them in the lab
c. Give one example of macromolecule
| Polysaccharides | Proteins | Nucleic Acids | |
| a. | Monosaccharides | Amino Acids | Nucleotides |
| b. | We mixed some solutions containing potassium iodide with polysaccharide solutions. | We used paper chromatography and gave the dried chromatographs to our lab instructor so he could be exposed to harmful ninhydrin as opposed to us. | We used paper chromatography and UV light on the dried chromatograph. |
| c. | Starch | Casein | Deoxyribonucleic Acid |
![]()
In the initial experiment, Section B pg. 17, where we were using potassium iodide
to identify polysaccharides, state which of the five soutions reacted positively,
which reacted negatively, and why.
| Potato Extract | Purple | Positive Reaction | |
| Potato=starch+skin+dirt | |||
| Starch Solution | Purple | Positive Reaction | |
| This is a no-brainer, it's starch. | |||
| Glycogen | Brown | Positive Reaction | |
| Humans store glucose in the muscles by converting it to Glycogen | |||
| Glucose | Orange | Negative Reaction | |
| A monosaccharide | |||
| Distilled Water | Orange | Negative Reaction | |
| A non-saccharide |
Since I really don't know my guess would have to be that the potassium iodide reacts with
polysaccharides and moreso maybe with greater concentrations of polysaccharide or
bigger polysaccharide molecules.
![]()
10/04/00
Prepare graphs of the data in tables 5.2, 5.4, 5.6, 5.8, and 5.10. Follow the
directions in appendix B and use the graph paper at the end of this
exercise. Turn the graphs in with this summary.
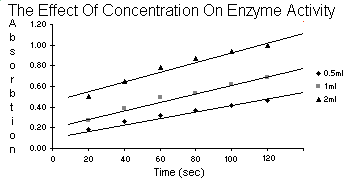
| Table 5.2 | Enzyme Concentration | ||
| 0.5ml | 1ml | 2ml | |
| 20 | 0.18 | 0.27 | 0.50 |
| 40 | 0.26 | 0.39 | 0.65 |
| Time 60 | 0.32 | 0.49 | 0.78 |
| 80 | 0.37 | 0.53 | 0.87 |
| 100 | 0.42 | 0.62 | 0.94 |
| 120 | 0.46 | 0.69 | 1.00 |
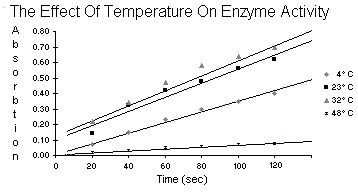
| Table 5.4 | Temperature | |||
4 C C
|
23 C C
|
32 C C
|
48 C C
|
|
| 20 | 0.07 | 0.14 | 0.22 | 0.02 |
| 40 | 0.15 | 0.32 | 0.34 | 0.03 |
| Time 60 | 0.23 | 0.42 | 0.47 | 0.05 |
| 80 | 0.30 | 0.48 | 0.58 | 0.06 |
| 100 | 0.35 | 0.56 | 0.64 | 0.07 |
| 120 | 0.40 | 0.62 | 0.70 | 0.08 |
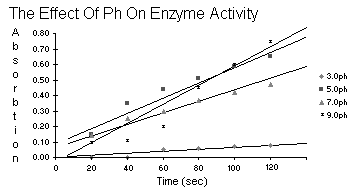
| Table 5.6 | ph Effect | |||
| 3.0ph | 5.0ph | 7.0ph | 9.0ph | |
| 20 | -- | 0.15 | 0.14 | 0.10 |
| 40 | -- | 0.35 | 0.25 | 0.11 |
| Time 60 | 0.05 | 0.44 | 0.30 | 0.20 |
| 80 | 0.06 | 0.51 | 0.37 | 0.45 |
| 100 | 0.07 | 0.59 | 0.42 | 0.60 |
| 120 | 0.08 | 0.65 | 0.47 | 0.75 |
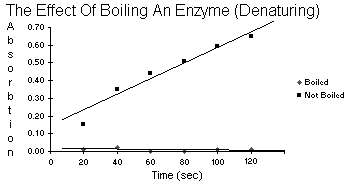
| Table 5.8 | Boiled vs. Not Boiled | |
| Boiled | Not Boiled | |
| 20 | 0.01 | 0.15 |
| 40 | 0.02 | 0.35 |
| Time 60 | 0.00 | 0.44 |
| 80 | 0.00 | 0.51 |
| 100 | 0.01 | 0.59 |
| 120 | 0.01 | 0.65 |
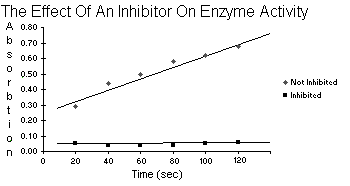
| Table 5.10 | Not Inhibited vs. Inhibited | |
| Not Inhibited | Inhibited | |
| 20 | 0.29 | 0.05 |
| 40 | 0.44 | 0.04 |
| Time 60 | 0.50 | 0.04 |
| 80 | 0.58 | 0.04 |
| 100 | 0.62 | 0.05 |
| 120 | 0.68 | 0.06 |
![]()
After reviewing your data plotted from table 5.2, give your reasons for
not rejecting or rejecting the hypothesis: peroxidase activity does
not vary with temperature. Describe what is meant by a
temperature optimum for an enzyme.
The enzyme in this experiment, peroxidase, when it was doing it's job turned the
water orange. We could measure how orange it was with the spectrometer.
If temperature were to have no effect, then accross the board for
temperature the activity of the enzyme would be similar and the
water similarly orange which would make the readings from the
spectrometer the same for each unit of time. But, they
aren't. Temperature being the ultramajor variable
proves the enzyme does not work at the same rate
at different temperatures.

I would imagine the temperature optimum is the temperature at which the enzyme
goes buck wild and works more than at any other temperature.
![]()
Review the plots of the data in table 5.4 and state your reasons for
not rejecting or rejecting the hypothesis: peroxidase activity
does not vary with pH. What is meant by a pH optimum
for an enzyme?
The enzyme in this experiment, peroxidase, when it was doing it's job turned the
water orange. We could measure how orange it was with the spectrometer.
If pH were to have no effect, then accross the board for pH the
activity of the enzyme would be similar and the water
similarly orange which would make the readings from
the spectrometer the same for each unit of time.
But, they aren't. pH being the ultramajor
variable proves the enzyme does not work
at the same rate at a different pH.

I would imagine the pH optimum is the pH at which the enzyme
goes buck wild and works more than at any other pH.
![]()
10/18/00
Define each of the following terms: diffusion, osmosis, facilitated
diffusion, and active transport. After you define each term,
compare and contrast it with the other three terms so as
to convince us that you understand the similarities
and differences among all 4. Be careful to
acknowledge your source(s) for the
definitions and other
information.
Diffusion: The net movement of dissolved molecules or other particles from
a region where they are more concentrated to a region where they
are less concentrated.
Osmosis: The diffusion of water across a selectively permeable membrane.
Facilitated Diffusion: Carrier assisted diffusion of molecules across a
cellular membrane through specific channels from a region of
higher concentration to one of lower concentration; the
process is driven by the concentration gradient and
does not require cellular energy from ATP.
Active Transport: The pumping of individual ions or other molecules across
a cellular membrane from a region of lower concentration to one of
higher concentration; this transport process requires energy,
which is typically supplied by the expenditure of ATP.
(definitions courtesy of the BIO 200 text's glossary)
| Diffusion | Osmosis | Facilitated Diffusion |
Active Transport |
|
| Diffusion | Same operation except diffusion is for all parties involved and osmosis has a selectively permeable membrane that keeps out the riff-raff. | Same operation except plain diffusion doesn't have a cellular membrane to cross. | Active transport is the opposite of diffusion plus a cellular membrane. | |
| Osmosis | Same operation except diffusion is for all parties involved and osmosis has a selectively permeable membrane that keeps out the riff-raff. | It looks to me like they are the same except the definition for facilitated diffusion says that the membrane to be crossed is specifically cellular. | Sounds similar as far as the membrane part goes, but osmosis is with the concentration gradient and active transport is against. | |
| Facilitated Diffusion |
Same operation except plain diffusion doesn't have a cellular membrane to cross. | It looks to me like they are the same except the definition for facilitated diffusion says that the membrane to be crossed is specifically cellular. | Complete opposites in respect to movement with/against concentration gradient and using/not using energy. | |
| Active Transport |
Active transport is the opposite of diffusion plus a cellular membrane. | Sounds similar as far as the membrane part goes, but osmosis is with the concentration gradient and active transport is against. | Complete opposites in respect to movement with/against concentration gradient and using/not using energy. |
![]()
Reproduce table 6.1 (p. 77, Dolphin manual) in your favorite word
processor and then complete it, using data from this lab
exercise. Answer the following quesions, which are
based on those in the lab manual.
| NaCl | ||||
Na SO SO |
||||
| Protein | ||||
| Starch | ||||
H O (weight) O (weight) |
||||
a. Which tubes served as a control in this experiment, and why/how?
The test tubes containing the "start" ingredients were our control group.
There would not be any mixing of the inside and outside
components. The control is the one that you would
compare the "end" concoctions with so as to see
if anything happened or not. The stock
solutions and the "start" solutions
should be the same.
b. Describe which ions were able to move through the dialysis
membrane. In which direction did they move, and why?
The small particles (i.e. all of the ions) were able to pass through the
dialysis membrane. The ions are very tiny as compared to the
starch and protein molecules which are too large to fit
through the small pores of the dialysis membrane.
In our case at the start of the experiment we had a pair of ions on
each side of the dialysis tubing and at the end of the
experiment we had all four ions on both sides of
the membrane. They moved because the two
pairs of ions diffused through the
membrane to an area of lower
concentration.
c. Were starch and the protein able to move through the dialysis membrane?
Why? Support your answer.
No, the starch and protein weren't able to pass through. The after tubes
that were tested did not test positive for the areas that they
did not begin in. They are too large to fit through the
pores of the dialysis tubing.
d. Did water move through the dialysis membrane? Why did it move and
how do you know that it moved?
Yes, water did move through the membrane. Water moves to equalize solute
concentration until it is stopped by some other factor or
accomplishes equilibrium. We can tell water moved
into the dialysis tubing because it's mass
increased at the end of the experiment.
![]()
Answer "Critical Thinking Question" #1, p.84 in the Dolphin lab manual.
1. If a person's blood volume drops due to injury or severe dehydration,
why do doctors administer isotonic saline intravenously instead
of pure water?
Low blood volume screws with blood pressure, and since replacing the blood is
dangerous or unnecessary we usually increase the volume by adding
various I.V. fluids. Blood cells are naturally bathed in
blood serum that has a controlled sodium cloride
concentration. Normal saline at 0.9% sodium
cloride does not interfere with that
natural concentration of
sodium cloride in the blood. If you were to add a stronger or weaker solution to
the blood you would create a hypotonic or hypertonic environment which
is bad. The correct answer is that doctors do not administer I.V.
fluids, nurses do. But, isotonic saline is used because it
matches the concentration level of sodium cloride in
the blood.
![]()
10/25/00
Use your favorite word processor software to generate and complete a
table like 7.3 in the Dolphin lab manual. Produce a line
graph (see p. 481, Appendix B, in the Dolphin manual
for a reminder of the difference between line
graphs and histograms) of the results of
table 7.3. Discuss and interpret the
results. You may earn 1 bonus
point by producing this
graph from software.
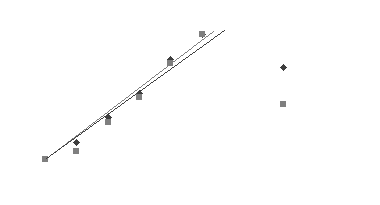
| Table 7.3 | Thermobar corrected data in millimeters | ||||||
I think our results got messed up somewhere, I donít think the azide-treated peas
were supposed to be producing as much if any oxygen. In our experiment
the treated peas caught right up with the untreated peas at the end.
![]()
Use your favorite word processor software, generate and complete a table
7.4 (p. 92 in the Dolphin lab manual). You must include your
calculations. These calculations may be done by hand, but
they must be legible, clearly organized and easily
comprehensible.
| Table 7.4 | |||||||
of O  Consumption Consumption(ml/min/g) |
|||||||

![]()
Answer "Critical Thinking Question" # 2, from p. 95 in the Dolphin lab
manual. Use specific real examples in your answer.
Describe what advantage organisms having anaerobic metabolism have over
those having only aerobic metabolism.
I suppose aerobic respiration is the way to go for an organism, so long as you have
molecular oxygen to work with because it extracts more energy per glucose.
But, if the process doesn't involve oxygen, that is one less thing to
worry about should oxygen resources get scarce. Anaerobes don't
need oxygen. E.g. anaerobic pathogens can thrive deep in
muscular tissue where aerobes would starve from lack
of oxygen.
Bonus Point: What do botulism, tetanus, gangrene, and "natural" pickles
have to do with this question?
They all employ anaerobic metabolism. The botulism, tetanus, and gangrene (gas)
bacteria all belong to the genus clostridium which are anaerobic,
gram-positive, rod-shaped, endo-spore forming bacteria. The
"natuaral" pickles do not get the lactic flavor removed as
well as regular pickles. I would assume the presence of
the lactic flavor advertised to be an indicator of
anaerobic metabolism of glucose in the cucumber
"natural" pickles are manufactured from.
Sources:
Genus Clostridium
Botulism
Tetanus
Gangrene
"Natural" Pickles
![]()
11/01/00
In your own words, describe what happens in each of the following stages of mitosis: prophase, metaphase, anaphase, telophase.
Prophase: Chromosomes condense in the nucleus and spindles form at the centrioles, then the nuclear membrane disappears and the spindles attach to the chromosomes at the centomeres.
Metaphase: The spindles pull the chromosomes to the equator of the cell.
Anaphase: The spindles split the chromosomes in half and pull them to their respective halves.
Telophase: The nuclear membrane and the nucleolus reappear and the cell constricts into two separate cells or builds a new cell wall.
![]()
Consider an hypothetical animal cell that undergoes mitosis. Assume the 2N number for this animal is 6. Carefully draw the form and arrangement of the chromosomes and of the rest of the cell during the stages of mitosis so as to convince us that you understand the process. (Hint: this might be a good time to find those colored pencils that you thought you might never use again...)
PICTURE
COMING
SOON
![]()
Using your favorite word processor, produce and complete a nice-looking version of Table 8.1 (p. 103) from the Dolphin lab manual. In your words, explain which phase of mitosis is longest, and what the evidence is for that.
Table 8.1 |
Determining duration of mitotic phases | ||
| Phase | Number Seen | % of Total | Length in Minutes |
| Interphase | 94 | 94 | 84.6 |
| Prophase | 2 | 2 | 1.8 |
| Metaphase | 1 | 1 | 0.9 |
| Anaphase | 2 | 2 | 1.8 |
| Telophase | 1 | 1 | 0.9 |
| Total | 100 | 100 | 90 |
As far as I know Prophase and Anaphase are the longest because of how many I saw versus how many cells I looked at. But the text leads me to believe Metaphase is the longest.
![]()
Bonus points: What two tissues in the adult human have the mitotically most active cells? Why are these tissues dividing so rapidly? Be sure to acknowledge your sources properly. [2 points maximum]
The fastest are the intestinal epithelial cells. Epithelial cells in general have many uses and arise from all three germ layers. One of their purposes is to protect other tissues from damage. It would be advantageous to be able to multiply quickly in an environment highly prone to damage. The intestines definitely qualify as that kind of environment.
Reference
The second fastest tissues are something I didnít know and couldnít locate the information on, but my guess would be embryonic cells because baby cells grow awful quick. Those or cancer.
![]()
