Unit 1 Study Guide
Introduction to Chemistry &
the Classification of Matter
E6. Compare the physical and chemical
characteristics of elements.
Activity #1 – Prelab
for “Who Kidnapped Roger Rabbit?”
Chromatography is a method for analyzing complex mixtures (such as ink) by separating
them into the chemicals from which they are made. Chromatography is used to
separate and identify all sorts of substances in police work. Drugs from
narcotics to aspirin can be identified in urine and blood samples, often with
the aid of chromatography.
Open Paper Chromatography. Write a paragraph summarizing the instructions.
Activity #2: Classification of Matter
Click on the links, define the following words, and take the quizzes:
MATTER
– definition
- mixture
– definition
- homogeneous
(solution) - definition
1.
2.
3.
4.
5.
- heterogeneous
– definition
1.
2.
3.
4.
5.
- substance
– definition
- element
– definition
periodic
table – definition
- compound
– definition
DO YOU GET IT?


3) quiz yourself – substance, homogeneous mixture (solution), heterogeneous mixture or heterogeneous solution?
a) yogurt
b) yogurt with real chunks of fruit
c) enamel-based paint
d) table salt
4) quiz yourself – write ALL letters of samples that fit the descriptions.


5)
Need a break?
Play Periodic
Table Breakout , The
First Twenty Elements Word Search, The First
20 Elements Jigsaw,
Activity #3: State
of Matter
Read A View
From a Distant Universe and answer these questions:
1)
Why do liquids and solids have a relatively fixed
volume (subject to small expansions and contractions due to temperature),
whereas the volume of a gas is much more variable?
2)
Why do crystalline solids have a fixed shape, whereas
liquids and gases adapt to the shape of their containers?
3) What is different about the way that liquids and gases adapt to their containers?
4)
What holds the molecules of a molecular liquid or solid
together? Why doesn't this same factor hold for gases?
5)
What were the earliest two chemical elements?
6) Why are these two elements so much rarer on Earth than they are in the universe as a whole?
Go to States
of Matter.
7) Write the correct phase next to the description.
a) ____________ rigid, fixed volume, fixed shape
b) ____________ definite volume, but no definite shape.
c) ____________ no fixed shape, no fixed volume
8) A phase diagram shows the temperature-pressure relations among the liquid, solid, and vapor states of a substance, Using the diagram below, what process is responsible for each of the following changes?

a) solid à liquid
b) liquid à solid
c) liquid à gas
d) gas à liquid
e) gas à solid
f)
solid à gas
Activity #4 - Prelab for Lab 2.2:
Mixture Separation
Open The Mixtures
Lab and perform the experiment. Fill
in the following table:
|
|
mixture |
separation mechanism |
physical
properties of each component that allow separation |
|
1 |
sand & iron
filings |
|
|
|
2 |
salad |
|
|
|
3 |
salt & water |
|
|
|
4 |
muddy water |
|
|
|
5 |
dust in air |
|
|
In your upcoming lab, you will be separating a mixture of sand, salt, iron filings and poppy seeds.
- What physical properties of each will help you to separate the components of this mixture?
- sand
- salt
- iron filings
- poppy seeds
- What methods might you use to separate them?
- In what order will you separate them?
Activity #5: Properties and Changes
Open
Properties
and Changes and navigate through the tutorial using the arrow keys at the
bottom. Define the following words
(clicking on the words in the tutorial will give you a pop-up definition from
the glossary):
1) physical property
a) definition
b) examples:
i) .
ii) .
iii) .
iv) .
v) .
vi) .
vii) .
2) chemical property
a) definition
b) examples:
i) .
ii) .
iii) .
3) physical change
a) definition
b) are/are not easily reversible (circle one)
c) examples
i) .
ii) .
4) chemical change
a) definition
b)
three
conditions that must be met for a chemical change
i)
.
ii)
.
AND
iii)
.
c)
examples
i) .
ii) .
iii) .
5) extensive property
a) definition
b) example
6) intensive property
a) definition
b) example
7) quiz yourself
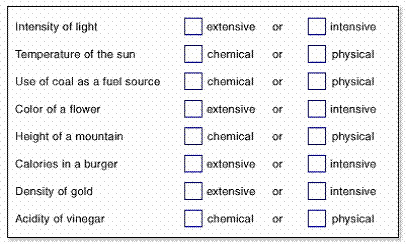



8) Another quiz – Open Physical Vs Chemical Change and classify the following as a physical (P) or chemical (C) change.
a) Frying an Egg _____
b) Vaporization of Dry ice _____
c) Boiling water _____
d) Burning Gasoline _____
e) Breaking Glass _____
f) Souring Milk _____
g) Compression
of a spring _____
Activity #6 –Evidence
for Chemical Changes (Class Demos)
- How do you know if a chemical change
has occurred? Visit Chemical
Change at Wikipedia and list ALL the
things you should be looking for:
- Define the following:
- endothermic
- exothermic
- precipitate
- For each of
the following demonstrations performed by your teacher, write down what
evidence you observed that shows a chemical change took place. For reactions that involve an energy
change, classify them as endothermic or exothermic.
- heating
magnesium
- mixing a
colorless solution of calcium chloride & sodium carbonate
- mixing a
pale yellow solution of iron chloride and a colorless solution of
potassium thiocyanate
- mixing
solid magnesium and a solution of hydrochloric acid
- mixing a
hydrochloric acid solution with a sodium hydroxide solutionmixing
solid barium hydroxide and solid ammonium chloride
Activity #7 –Measuring Matter
1) Open
Measuring
Matter and define:
a) Inertia –
b) Mass –
c) Conservation of Mass –
d) Volume –
e) Density –
f) Weight –
g) Mole –
2) Open Measuring Matter Crossword, do the puzzle online, check it, & fill it in below:
Review Activities:
- Go to Introduction to Chemistry Vocabulary for the clues to this crossword puzzle. Do it online and then fill in your answers below.


- Fill in this chart with the words below (have your teacher check this when you are done):
|
matter |
substance |
element |
|
homogeneous |
heterogeneous |
chemically |
|
physically |
solution |
alloy (bronze) |
|
compound |
quartz |
granite |
|
gold |
mixture |
|

UNIT 1 CHECKLISTS
Unit 1 Homework (check off when done and passed in):
_____ Science Help Online Worksheet 1-4a Classification of Matter
_____ Science Help Online Worksheet 1-8a Elemental Names and Symbols
_____ Activity
2.3: Elemental Test
_____ Science Help Online Worksheet 1-5a Properties of Matter
_____ Science Help Online Worksheet 1-5d Changes in Matter
Unit 1 Web Activities (check off when done and passed
in):
_____ Web Activity 2.2, 2.4 - Distinguishing Elements, Compounds & Mixtures
_____ Worksheet
2.2, 2.3 - More Elements Compounds and Mixtures
_____ Density
- Virtual Lab
Unit 1 Labs (check off when done and passed in):
_____ Who Kidnapped
Roger Rabbit?
_____ Lab 2.2: Mixture Separation
_____ Experiment 2.3 – 2.4 Electrolysis of Water
_____ Lab Addition to Ch. 2 - Density
Unit 1 Charts and diagrams:


