 |
Water Analysis |
 |
Why do we need to analyze water?
If water is badly polluted-- like raw sewage--- it might be obvious from its appearance or odor.
It might be colored or turbid (cloudy), or have solids, oil or foam floating on it.
It might have a rotten odor, or smell like industrial chemicals.
A lot of dead fish floating on the surface of a lake would be a clear sign that something was wrong.
But many harmful-- and beneficial-- materials in water are invisible and odorless. In order to go beyond the obvious, to determine what materials are in the water, and how much, we need to be able to conduct chemical or microbiological analyses.
Analysis of a natural body of water will tell us how clean or polluted it is. If there is damage to wildlife, the measurements will help pinpoint the cause-- and the source. In a wastewater treatment plant, analyses are necessary for monitoring the effectiveness of the treatment processes. In the United States, the Clean Water Act requires wastewater dischargers to have permits. These permits set limits on the amounts of specific pollutants which can be discharged, as well as a schedule for monitoring and reporting the results. Usually, the reports must be filed monthly, while the measurement frequency for a particular parameter (measurable property) can run anywhere from "continuously" to just once a year. Only standard analytical procedures specified in the "Code of Federal Regulations" may be used, so that the government agencies can feel reasonably confident that results from different laboratories are comparable.
Similar considerations apply to drinking water. The purity of the water we drink is of more concern to the average person than the quality of the wastewater discharged by the sewage plant. But we should not forget that in many places, especially along a river, one town's wastewater discharge may be part of the next town's water supply...
There are two aspects to water analysis that we need to consider:
- what substances or organisms are we interested in testing for-- and why?
- what procedures and equipment do we use to make the measurements, and how do they work?
Let's look at the "procedures and equipment" first:
(If you want to read about the "substances and organisms" first,
click here,-- or here for no-frames version-- but the procedures on that page refer to methods discussed below.)
Analytical Methods 
Water analyses are done by several methods. The most common types of measurements are gravimetric (weighing), electrochemical (using meters with electrodes) and optical (including visual). Instrumental methods are becoming increasingly popular, and instrumentation is getting "smarter" and easier to use with the inclusion of microprocessors. In the simplest case, a sample may just be placed in an instrument and a result read directly on a display. More often some physical separation technique or chemical procedure is needed before a measurement is made, in order to remove interferences and transform the analyte-- the target of the analysis-- into a form which can be detected by the instrument.
Since even raw sewage is generally more than 99.9% water, most environmental analyses are measuring very low concentrations of materials. The results of these measurements are usually expressed in the units "milligrams per liter," abbreviated as mg/L. Since a milligram is one thousandth of a gram, and a liter of water weighs about a thousand grams, a mg/L is approximately equal to one part per million by weight. A part per million ("ppm") is only one ten thousandth of one percent. For toxic metals and organic compounds of industrial origin, measurements are now routinely made in the part per billion (microgram per liter) range or even lower. At such low levels, sensitive equipment and careful technique are clearly necessary for accurate results. Avoiding contamination of the sample and using methods which prevent interferences from other substances in the water are crucial requirements for successful analyses.
Separation Techniques:
Some measurements require separating the analyte from other substances in the water which may interfere with the measurement. Some measurements even require separating the analyte from the water entirely. Separation techniques include:
- Filtration: The water is passed through a fine-pore filter which can be made of paper, glass fibers, a cellulose acetate membrane, etc. Filtration through a filter of some agreed-upon standard pore size can be used to separate "suspended" from "dissolved" portions of the analyte. The analyte may be the suspended matter which is captured on the filter-- or the filter may be used to clarify the water for analysis of a dissolved material. Often, the filtration is assisted by applying a vacuum below the filter, which is supported on a porous holder in some type of funnel.
- Distillation: If the analyte can be boiled out of the water, or along with the water, then the vapors can be cooled and re-condensed or trapped in a liquid form in a different container. This way the analyte can be removed from the interfering substances in the original water sample. Often the sample is made acidic or alkaline, or treated chemically in some other way before distillation, to convert the analyte into a volatile (easily evaporated) form, and to immobilize or neutralize interfering substances.
- Extraction: Some analytes may be much more soluble in an organic solvent than in water. If the solvent does not mix with water, and the sample is shaken with portions of the solvent, almost all of the analyte may be transferred from the water into the solvent, leaving interfering substances behind. This is known as a "liquid-liquid" extraction. The analysis may be completed using the organic portion. There are also continuous versions of this process for use with liquid or with dry samples.
Another type of extraction is called "solid-phase extraction." In this kind of procedure, the sample is passed through a column or filter containing a powdered or granulated material which retains (adsorbs) the substances of interest and allows other types of dissolved materials to pass through. Then a solvent, or an acid or alkaline solution, can be passed through to de-sorb and redissolve the analytes, a process known as elution.
Either type of extraction can also be used to concentrate the analyte into a smaller total volume, which increases the sensitivity of the analysis. This can be true for distillation or filtration, as well.
Measurement Techniques:
- Gravimetric analysis or, simply, weighing:
Analytical balances routinely used for gravimetric analysis are sensitive to one tenth of a milligram, or one ten-thousandth of a gram. Most laboratories use electronic balances with direct digital readouts. For a measurement of the milligrams per liter of solids in the water, a measured volume of sample can be dried in a tared (pre-weighed) dish; the dish plus solids are weighed after the water has evaporated off; the weight of solids is calculated by subtraction, and the concentration figured by dividing the weight of solids by the volume of the sample. For a filtered sample, the tared filter itself is dried along with the solids it captured, and the suspended solids (those captured on the filter) calculated in the same way. In some chemical analyses, a precipitate is formed by reacting the analyte of interest with another chemical reagent (reacting chemical); then the precipitate can be filtered, dried, and weighed as a suspended solid. This type of analysis is more common with water solutions that are more concentrated than environmental samples, though, such as chemicals purchased for use in water or wastewater treatment.
- Electrochemical:
The outer portions of all atoms and molecules consist of "shells" of electrons, and all chemical reactions involve interactions with these outer electrons-- sharing or transfer, or something in between. It is not surprising,, then, that electricity and chemistry are interrelated (just think of batteries), and that electrical measurements can be used to detect and determine some substances of interest. The procedures involve placing electrodes in a water sample and measuring either an electrical potential (voltage), in millivolts, or a current, in milliamperes, which is related to the concentration of analyte. Depending on what they are designed to measure, electrodes can be simple pieces of metals such as gold, silver, platinum, copper, etc.; or they may be elaborate systems with semi-permiable membranes and several internal electrodes and filling solutions. The instrumentation may be capable of reading out directly in concentration units. Usually some sort of calibration procedure is necessary, using one or more standard solutions of known concentration.
- Colorimetry or spectrophotometry:
This method involves measuring the intensity of a color in a solution and relating it to the concentration of the analyte. While some materials of interest are already colored, most of these analyses require the analyst to add some chemical reagents (reacting chemicals) to a sample to produce a characteristic color.
The simplest type of measurement is visual comparison of the intensity of the color to a set of color standards which represent various concentrations of the analyte. While this is method does not require any expensive equipment, color perception is rather subjective-- and many people have some degree of color-blindness.
A more precise measurement can be made using a colorimeter. A colorimeter is a device consisting of 1) a light source, which can be as simple as tungsten-filament light bulb; 2) some optics for focusing the light 3) a colored filter, which passes light of the color which is absorbed by the treated sample; 4) a sample compartment to hold a transparent tube or cell containing the sample, 5) a light-sensitive detector, like the light meter on a camera, which converts the light intensity into an electric current, and 6) electronics for measuring and displaying the output of the detector. Some colorimeters may be designed to read out directly in concentration units, while others may show the results in units of light absorbance which need to be compared to a calibration curve. (An interesting point is that the filter is not the same color as the solution being tested, but rather the complementary color. We want to use a filter which transmits light of the color which the solution absorbs. A yellow solution looks yellow because it absorbs blue light, so a blue filter would be used.)
If we want to get more precise and more interference-free measurements, we can use a spectrophotometer. This is very similar to a colorimeter, except that instead of using a filter to select the color of light to pass through the sample, we instead break the white light up into a rainbow (spectrum) of colors using a prism or a diffraction grating. The light is passed through a narrow opening (slit) before reaching the sample. By rotating the prism or grating, the color {"wavelength") of light can be selected more precisely and we can better match the color with that absorbed by the sample. The principle is shown in the diagram below.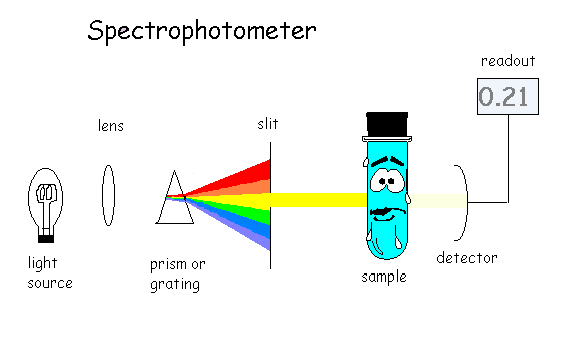 Needless to say, spectrophotometers cost more than colorimeters, and are likely to be more delicate and less portable, as well. While many tests are done using visible light, some analyses also make use of the invisible ultraviolet or infrared portions of the spectrum. Scanning spectro photometers can also be used to identify some types of analytes by the wavelengths or colors of the light they absorb.
Needless to say, spectrophotometers cost more than colorimeters, and are likely to be more delicate and less portable, as well. While many tests are done using visible light, some analyses also make use of the invisible ultraviolet or infrared portions of the spectrum. Scanning spectro photometers can also be used to identify some types of analytes by the wavelengths or colors of the light they absorb.
There is a variation of this type of testing, usually referred to as atomic spectroscopy, which is used mostly for trace metal analysis. The sample is converted to a gas by one of several methods-- usually involving heating. Then the light from a lamp containing the same metal is passed though the gas and the absorbance measured just as with a liquid sample (atomic absorption spectrophotometry). Alternatively, the intensity of the light emitted from the heated atoms of the metal in the gas can be used as a way of measuring the concentration (atomic emission spectrophotometry). A very popular atomic emission method in use today is called inductively coupled plasma spectrometry. The sample is carried in a stream of argon gas surrounded by coils which emit radio frequency energy that converts some of the gas into a very hot, ionized (electrically charged) form. An advantage of this method is that many elements can be measured simultaneously, or in rapid succession.
- Titration:
Titration depends on using a well-defined chemical reaction to measure the amount of a standard solution needed to react with certain amount of the sample. A known volume, such as 100 mL, of sample is placed into a flask or beaker. The standard reagent is dispensed from a graduated tube called a burette so the volume used can be measured. The "end point" of the reaction is usually determined by observing a color change in an indicator solution, which is added to the flask before the start of the titration. End points are also often determined using electrochemical equipment. Once we know how much of the standard reagent was needed, we can calculate the amount of the analyte that is in the sample, because the reaction will always use the same proportion of the two materials. A common example is measuring the concentration of an acid by titrating with a standard base, such as sodium hydroxide.
- Chromatography:
This technique got its name, which means "color picture", because it was first used to separate colored pigments from a single spot on a piece of paper. A solvent, such as alcohol, is allowed to move slowly across the paper, and the different components of the pigment travel at different rates. The result is a series of separated spots of different colors. They move at different rates because of differences in the pigments' relative attraction to the paper (the "stationary phase") and their solubility in the solvent (the "mobile phase"). This principle is used in modern instrumentation to separate mixtures of organic chemicals or inorganic ions. The components can be identified by their retention times,-- i.e., how long it takes them to pass through the instrument-- and detectors can be used to measure the amount of each component.
In gas chromatography, (or, simply, "GC") the mixture of substances is injected into a narrow, coiled column, several feet long, made of an inert material like glass, silica or stainless steel. The sample has usually been extracted into an organic solvent and concentrated by evaporation as a pretreatment step. The column may be filled with an oil-coated, powdered mineral, which forms the stationary phase. In the narrower capillary columns, the stationary phase is bonded directly to the wall of the tubing. The columns are usually contained in an oven, which may be programmable to raise the temperature at a controlled rate over time. Heating the column allows analysts to use this technique on many substances which are not gases at room temperature, including solvents and toxic chemicals like pesticides and PCB's. A continuous flow of an inert gas, such as argon, helium, or sometimes nitrogen, carries the evaporated mixture through the column. The substances are detected as they exit the column, usually by a technique that converts them into ions (electrically charged atoms or molecules), although one method uses heat conduction. The ions are produced by means such as flames, ultraviolet light, or radioactive materials. They are detected by being attracted to charged plates, where they produce an electrical current proportional to the amount present. The output of the detector usually is shown as a chart of "peaks" vs. time, called a chromatogram, often with the retention time and the intensity of the peak printed out. The retention time is used to identify the substance, while the height or area of the peak is used to quantify its concentration. A more positive identification is possible using a mass spectrometer (see below) as the detector.
For substances which cannot easily be vaporized because of high boiling point or instability at higher temperatures, there is a liquid version of this technique know as HPLC (High pressure or high performance liquid chromatography). Organic solvents are used as the mobile phase. Ultraviolet (UV) light absorption is often used for detection. Herbicides and pharmaceuticals are common types of substances analyzed by this technique. Another variation of LC is ion chromatography, (IC), where the target analytes are charged inorganic or organic substances. The mobile phase is an aqueous (water-based) solution, and the stationary phase is made up of an ion exchange resin. The detectors usually measure electrical conductivity, although UV absorption can also be used. This technique can be used to measure the concentrations of several important inorganic anions, such as fluoride, sulfate, phosphate, and nitrate all in one analysis
- Mass Spectrometry:
In a mass spectrometer, an ionized vapor is passed between magnets or radio frequency coils which separate the ions by mass (actually by charge to mass ratio). The pattern produced is characteristic of the particular substance, which can be identified by comparison with computerized "libraries" of mass spectra. While the instrumentation can be used alone, for environmental analyses it is usually used in tandem with another technique. Used as a "detector" for gas chromatography ("GC-MS"), it can positively identified components which have already been separated from a mixture. There is some use with liquid chromatography, as well (LC-MS). As a detector for metal ions produced in an ICP (see above), it provides very high sensitivity and is being used to determine very low levels of metal in drinking water, and may soon be approved for wastewater effluents and receiving waters.
What do we test for, why, and how?

(click here for no-frames version...)
This page hosted by
 Get your own Free Home Page
Get your own Free Home Page
This page accessed  times since Nov. 23, 1997.
times since Nov. 23, 1997.





 Needless to say, spectrophotometers cost more than colorimeters, and are likely to be more delicate and less portable, as well. While many tests are done using visible light, some analyses also make use of the invisible ultraviolet or infrared portions of the spectrum. Scanning spectro photometers can also be used to identify some types of analytes by the wavelengths or colors of the light they absorb.
Needless to say, spectrophotometers cost more than colorimeters, and are likely to be more delicate and less portable, as well. While many tests are done using visible light, some analyses also make use of the invisible ultraviolet or infrared portions of the spectrum. Scanning spectro photometers can also be used to identify some types of analytes by the wavelengths or colors of the light they absorb.







 times since Nov. 23, 1997.
times since Nov. 23, 1997.