   What is Laser What is Laser
 The way Laser Works The way Laser Works
 The Power range of Laser The Power range of Laser
 Feedback Feedback
 Applications of Laser Applications of Laser
 Hazards with Laser Hazards with Laser
 Resources & Different Web Sites Resources & Different Web Sites
 Test Yourself Test Yourself
|
|---|
Different Types of Laser
There are many ways to define the type of laser.
Based on its pumping scheme a laser can be classified as
 Optically pumped laser
Optically pumped laser
 Electrically pumped laser
Electrically pumped laser
On the basis of the operation mode, laser fall into classes of
 Continuous Wave Lasers
Continuous Wave Lasers
 Pulsed Lasers.
Pulsed Lasers.
According to the materials used to produce laser light, lasers can be divided into three categories :
(i) Gas Laser:
Gas lasers generally have a wide variety of characteristics. For example , some gas lasers emit feeble power below 1mW, but other commercial gas lasers emit power of the order of kilowatts. Some lasers can emit continuous beam for years, others emit pulses lasting a few nanoseconds. Their outputs range from deep in the ultraviolet through the visible and infrared to millimetre waves. Examples are:
(a). Helium- neon (HeNe)

laser (most frequently use) with its familiar red beam (Fig.6). The laser medium is a mixture of helium and neon gases. An electrical discharge, in the form of direct current or radio frequency current, is used to excite the medium to a higher energy level. The pumping action takes place in a complex and indirect manner. First the helium atoms are excited by the discharge to two of the excited energy levels (Fig.7). These two levels happen to be very close to the 3s and 2s levels of the neon atoms. When the excited helium atoms collide with the neon atoms, energy is exchanged, pumping the neon atoms to the respective levels. The atoms at the neon 3s level eventually drops down to the 2p level, as a result of stimulated emission, and light of wavelength 632.8 nm is emitted. The atoms at the 2s level, on the other hand, drops to the 2p level by emitting light at 1.15 nm. However , the atoms at the 3s level may instead drop down to the 3p level, by emitting light at 3.39 mm. 632.8nm is in the visible range.
Figure 6 : He-Ne Gas Laser

(b). Gallium Arsenate (GaAs) Laser
(ii). Solid state laser:
A solid state laser is one in which the atoms that emit light are fixed within a crystal or a glassy material.The first laser invented by Maiman in 1960 , the ruby laser, was a solid state laser. The atoms that emit light in solid state lasers are dispersed in a crystal or a piece of glass that contains many other elements. The crystal is shaped into a rod, with reflecting mirrors placed at each end. Light from an external source (such as a pulsed flash lamp, a bright continuous arc lamp, or another laser) enters the laser rod and excites the light-emitting atoms. The two mirrors form a resonant cavity and the inverted population in the laser rod, provided the feedback needed to generate a laser beam that emerges through the output mirror.
As the photons traverse the crystal, they stimulate the emission of additional photons until enough energy is available for a pulse of photons to break through the thinly mirrored end on the right of the laser crystal.
The Nd:YAG laser is a good example of the most commonly used solid state lasers. The laser medium is made up of yttrium- aluminium-garnet, with trivalent neodymium ions present as impurities. The laser transition involved corresponds to a wavelength of 1.06 mm, in the near infrared region (Fig.8,9).
(iii). Semiconductor Laser:
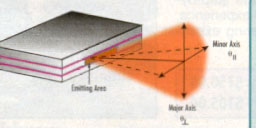

A unique ,and perhaps the most important, type of laser in terms of opto- electronics applications is the semiconductor laser. It is unique because of its small dimensions (mm x mm x mm) , and its natural integration capabilities with micro electronic circuitry, Furthermore, the light amplification by the process of stimulated emission is not exactly in the form that we have described before.
A semiconductor laser uses special properties of the transition region at the junction of a p-type semiconductor in contact with an n-type semiconductor. In semiconductor materials, because of the extensive interaction of energy between atoms, the energy levels form bands. Energy band diagrams for an n-type and a p-type semiconductor are depicted in Fig.10. The energy gap between the valence band and the conduction band is designated by Eg and is measured in electronvolts,e.g., the Fermi level Ef is the level that divides the occupied from the unoccupied levels.
In a p-n junction, as shown in Fig.11, the energy levels readjust in accordance with thermodynamics so that the Ef band is the same through the junction. The valence band Evv and the conduction band Ec of the p-type semiconductor are higher than the corresponding bands of the n-type semiconductor. If a positive voltage is applied on the p side (the so-called positive bias), the electrons on the n side will be attracted by the applied voltage and will cross into the junction region. There they recombine with the holes that have been pushed into the junction region by the positive bias. This process will continue as long as the external circuit is on, because the electrons and holes that have recombined are continuously replenished.
When the electrons and holes recombine, they emit energy in the form of photons. The junction transition region in which this takes place is therefore the source of radiation, and may be viewed as equivalent to the E2 and E1 transition levels which we discussed earlier.
To obtain stimulated emission and amplification from this region, the equivalent of the population inversion needs to be created, for which a high density of electrons and a high density of holes must exist simultaneously in the junction region. To achieve this, heavily doped p-n junctions are used in semiconductor lasers.
Fig.12a shows the resultant energy levels. When a positive bias is applied on the p-side, there is a transition region with a high concentration of electrons and holes, as shown in Fig.12b. This region serves as a population inverted medium, which amplifies the radiation emitted within it through electron - hole recombination. A p-n junction semiconductor laser is illustrated schematically in Fig.13. The shaded area is the transition region where the laser action takes place. This region is about 1-2 mm thick, and tens of micrometers long. As a result, the emission is squeezed into a thin plane, leading to an elliptical cross-section of the beam.
(iv) Other Laser devices:
Ion and Metal Vapour Laser:
Operated at high temperatures to keep the metals (e.g., copper, gold etc.) vaporized and produced laser light in the infrared, visible or ultraviolet regions. They are excellent sources of short, high-intensity laser pulses at very high pulse-repetition rates.The mixture of metal vapour and noble gases are excited by electrical discharges. Example is copper vapour lasers which produces green and yellow light from a mixture of copper vapour with helium and neon.
Carbon Dioxide Laser.
The excimer Laser.
The liquid (dye) Laser :
Dye lasers use liquid organic dyes. Dye lasers can produce a broad and almost continuous range of colors, mainly in the visible part of the spectrum. With proper optical system any colour can be selected or tuning from one colour to another can be done. That is why dye lasers are particularly suited for applications in which a precise colour is required. Usually another laser source e.g., copper vapour lasers are used to excite the dye.
The Free Electron laser.
   
 Hazards with Laser Hazards with Laser
 Resources & Different Web Sites Resources & Different Web Sites
 Test Yourself Test Yourself
 Feedback Feedback
|
|---|
