In fact, an iceberg is nothing but a giant ice cube. When you throw an ice
cube in a glass of water, it will float. Floating has nothing to do with size
but all with weight. So the floating of an ice cube is just the same as floating
of an iceberg. How is this possible. 
When you take 1 litre (= 1 dm3=1000 cm3) of water and weigh it, you will see
that 1 liter of water weighs 1 kilogramm, when you weigh 1 liter of icecubes
you will find that the icecubes weigh less. You can try it with a box filled
with water first and then with icecubes, although you'll need a very precise
weighing device.
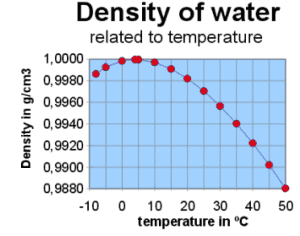
In the graphic you see the following:
From 50º C to 4º C water gets heavier (i.e. the density rises) from
0.988 gramms per cm3 (=988 grams per dm3) to 1.000 (=1000 grams per dm3).
When water gets colder than 4º C it becomes lighter, at 0º C it becomes
ice and it weighs 0.9998425 grams per cm3, lets say 1 grams less per dm3.
The water temperature in the polar areas is 4º C at maximum, so icebergs
will float.
Another aspect is the salinity of the seawater. Salinity makes water heavier.
Salty ocean water weighs 1.025 grams per cm3, making the iceberg even easier
to float.
What follows is a detailed article from the encycopedia Britannica:
In the liquid state, most water molecules are associated in a polymeric structure--that is, chains of molecules connected by weak hydrogen bonds. Under the influence of thermal agitation, there is a constant breaking and reforming of these bonds. In the gaseous state, whether steam or water vapour, water molecules are largely independent of one another, and, apart from collisions, interactions between them are slight. Gaseous water, then, is largely monomeric--i.e., consisting of single molecules--although there occasionally occur dimers (a union of two molecules) and even some trimers (a combination of three molecules). In the solid state, at the other extreme, water molecules interact with one another strongly enough to form an ordered crystalline structure, with each oxygen atom collecting the four nearest of its neighbours and arranging them about itself in a rigid lattice. This structure results in a more open assembly, and hence a lower density, than the closely packed assembly of molecules in the liquid phase. For this reason, water is one of the few substances that is actually less dense in solid form than in the liquid state, dropping from 1,000 to 917 kilograms per cubic metre. It is the reason why ice floats rather than sinking, so that, during the winter, it develops as a sheet on the surface of lakes and rivers rather than sinking below the surface and accumulating from the bottom. As water is warmed from the freezing point of 0 to 4C (from 32 to 39F), it contracts and becomes denser. This initial increase in density takes place because at 0C a portion of the water consists of open-structured molecular arrangements similar to those of ice crystals. As the temperature increases, these structures break down and reduce their volume to that of the more closely packed polymeric structures of the liquid state. With further warming beyond 4 C, the water begins to expand in volume, along with the usual increase in intermolecular vibrations caused by thermal energy.
click
HERE for more info on the ARCTIC and ICEBERGS