English version |
Polymolecular structure of water |
|
Russian coding:
|
Water is weird. It's a liquid when it ought to be a gas, it expands when it should contract, and it dissolves just about everything it touches -- given enough time. Yet without water's weirdness, Earth would be just another lifeless snowball in space.”
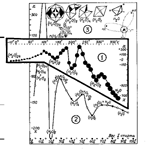
There are many different theories about water structure, but still they are not good enough to explain the strange behavior of this important and enigmatic substance. I started to think about water structure a long time ago, back in the sixties. I want to present some of my ideas here. As everyone knows, the composition of a water molecule is quite simple – one atom of oxygen and 2 of hydrogen (fig.1 a). The molecule has two local negative electric charges on the oxygen atom and one positive on each of the 2 hydrogen atoms. Local electric charges bend the molecule in such a way, that its shape is pretty close to a tetrahedron (fig. 1 a). Every apex of the tetrahedron has a local charge, as a result of dipole moment. Trying to understand the structure of water, I started to play with plastic tetrahedrons trying to place them in compact, energy-gaining complexes. I found they could be assembled into conglomerates of 24 molecules (fig1a). I suggest that (H2O)24 is the main component of pure water at room temperature. When water is heated under pressure, or titrated by ethanol, 24-mers must be broken down to smaller conglomerates, such as (H2O)8, (H2O)4, (H2O)2 and H2O (fig1b,c). To check this I devised an algorithm, shown on the abscissa (I don’t want explain it here) and found, that water behavior has peaks and troughs according to my hypothesis (fig1 b,c). The shape of snowflakes can also prove my model of water polymers. (H2O)24 conglomerates have 6 open in a single plane “windows” each of them is oriented 60o from the previous. In cold air (H2O)24 particles start to attach to each other window to window, forming thin, fractal-like structure with 6n symmetry. This explains why snowflakes have 6n (60 degrees) symmetry. My theory helps us to understand many of the weird qualities of water, such as
If you want to discuss these ideas, or to learn more about them, you can contact me by e-mail, or snail mail. |