

II. IDENTIFICATION AND CHARACTERIZATION OF GIARDIA ENDOMEMBRANES.
Despite there having already been 30 years of research
on Giardia's subcellular structure, I and Rad Gupta have provided the first definitive
evidence for the presence of endoplasmic reticulum (ER) in this organism (Reference 7). You may click on electron microscopic (EM) pictures or you can read the actual publication. Permission to make these items available on the Internet was obtained from the Journal of Cell Science and the Company of Biologists Ltd.
1.Low magnification EM of Giardia.
2.Higher magnification EM showing ER membranes. The ER is labelled with antibody against the ER protein called Bip. The antibody is bound to colloidal gold markers, which in EM appear as black dots.
3.Read the actual publication.You will need Adobe Acrobat Reader.
For a review of this study, see the ‘Headlines' article which
appeared in Trends in Cell Biology (7). This work depended on (i) the
use of cryotechniques to preserve endomembranes and (ii) raising an antibody
against recombinant giardial Bip, the hsp70 homolog resident in ER in higher
eukaryotes, to serve as a definitive molecular label. This work confirmed
a central dogma in cell biology, namely that the endomembrane system and
nucleus co-evolved in the same evolutionary event and that all eukaryotic
cells would possess both. In addition to characterizing the structural
organization of the ER, this study also identified membrane systems
in trophozoites which appeared to represent a Golgi apparatus. Although
a Golgi apparatus was previously reported by others to form uniquely during
encystation (4), our studies led us to conclude that a Golgi apparatus
is present throughout the life cycle but in functionally different forms.
Further confirmation of this will require molecular labels which specifically
identify the Golgi. Giardia should prove to be an excellent model system
for studies into developmental changes in Golgi structure and function,
vesicular transport and regulated versus constitutive secretion, with the
changes that occur during encystation and excystation serving as models
of cell differentiation.
III. IDENTITY AND ORGANIZATION OF THE GIARDIA CYTOSKELETON.
The processes of cytokinesis and cell differentiation
are driven by dramatic restructuring of cytoskeletal structures. I have
published a detailed study of the organization of the microtubule-based
cytoskeleton in trophozoites (8) which also included a partial investigation
of postranslational tubulin modifications (postranslational modifications are chemical reactions that change the biological activity and/or localization of a protein). In the case of tubulin, postranslational modifications may alter the assembly/disassembly dynamics of microtubules. You can click on some fluorescence or electon microscopic pictures:
1.Immunofluorescence micrograph of Giardia microtubules. Cells were labeled with a fluorescent antibody against acetylated tubulin.
2. Electron micrographs of flagellar axonemes and the median body. Microtubules in these structures, which look like railroad tracks when viewed longitudinally (A and B) or as circles when viewed in cross section (C),are labeled with antibody against acetylated tubulin bound to colloidal gold markers, which appear as black dots.
3. Flagella microtubules in cross section Giardia flagella have the typical 9(2)+2 axonemal structure found in higher eukaryotes (nine sets of two microtubules arranged in a circle with two microtubules in the center) and not a more primitive version.
4. Longitudinal sections of cytoplasmic axonemes Kinetosomes from which microtubules are nucleated are seen at the top of part B.
5. Electron micrographs showing labeling of the adhesive disk with antibody to acetylated tubulin. The adhesive disk is a microtubule-based structure only found in Giardia.
Since postranslational modifications of tubulin are known to significantly alter microtubule assembly-disassembly
dynamics, further investigation of these throughout the life cycle are
warranted. Thus far, the cytoskeleton of trophozoites appears to be a rather
stable structure, with all microtubules being acetylated . Future work should include examination of cytoskeleton
dynamics during encystation and excystation, when dramatic structural rearrangements
occur. Moreover, mitosis in Giardia has not yet been adequately described.
Since it is no doubt the simplest mitosis in all eukaryotes, Giardia may
be an excellent model system for understanding mitosis in general. The
mitotic spindle is also a potential target for therapeutic intervention
in cases of giardiasis, and tests of anti-mitotic drugs would benefit from
an understanding of Giardia's mitotic spindle physiology.
IV. DID GIARDIA LOSE MITOCHONDRIA?
A large number of protists, including Giardia, lack mitochondria. In the past this has been taken as evidence that these organisms existed before the endosymbiosis event which led to mitochondria, and hence were more primitive than other protists. In endosymbiosis, a theory made popular in its modern version by Dr. Lynn Margulis 30 years ago, oxygen respiring bacteria invaded a host cell and formed a permanent relationship living within it, evolving into mitochondria. This endosymbiotic event is thought to have occured more than 1000 million years ago [our planetary system formed 4600 million years ago; the first bacterial cell appeared 3900 million years ago; the first protists appeared 2000 milllion years ago; man's ancestors appeared 4 million years ago]. Mitochondria in cells actually still look like bacteria and grow and divide at their own pace. They even have their own DNA, although most genes over time have been transferred to the nucleus.
Despite the fact that Giardia lacks mitochondria, I and Rad Gupta published work showing the presence of a protein related to mitochondrial hsp60 in Giardia (9). The evidence included biochemical immunoblot detection of a protein of the correct molecular weight and both immunoflourescence and electron microscopic localization of reactivity at discrete sites in the cytoplasm.
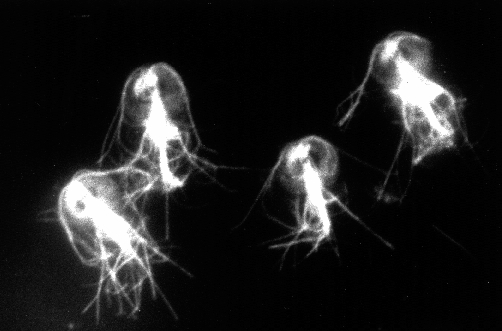
1.Immunofluorescence micrograph of Giardia.Cells were labeled with antibody against mammalian hsp60. Hsp60 is considered a mitochondrial protein in higher eukaryotes. The fluorescent dots throughout the cytoplasm are suggestive of organelle labeling.
2. Double label immunofluorescence.Hsp60 antibody labeling in A is compared with anti-tubulin labeling of the same cells in B. Some microtubule structures are identified in B: MB=median body, AF=anterior flagella, AD=adhesive disk.
Electron microscopic localization of hsp60 showed that hsp60 labeling was in the cytoplasm and was not associated with any type of membranous structure (not shown). To explain the findings we suggested that Giardia originally had mitochondria
but lost them in evolution. More recent studies in the higher protist Trichomonas
vaginalis, which contain hydrogenosomes but no mitochondria, showed molecular
evidence for the presence of mitochondrial heat shock proteins within hydogenosomes (the hydrogenosome is a double membraned redox organelle found in certain anaerobic protists). Palmer et al (10) have reviewed this work. The results led to the
suggestion that hydogenosomes evolved (or de-evolved, depending on how
you look at it) from mitochondria by a process of reductive, as opposed
to acquisitive, evolution. Since Giardia has been regarded as the most primitive eukaryote in existence, Palmer et al (10) also cite our work as evidence from diplomonads to support
the idea that the earliest eukaryotic cell contained mitochondria which
were subsequently lost. Thus, the timing for the endosymbiotic event that
gave rise to mitochondria is currently being pushed backwards. We are faced with the possibility that no representative of the premitochondrial stage of eukaryotic evolution may be alive today. The endosymbiotic event that gave rise to mitochondria in fact may have occurred as far back as the very origin of the first eukaryotic cell. The key to resolving this issue
would be to obtain further molecular data in Giardia. The cloning of a variety of mitochondrial proteins will be necessary. It may be very difficult, however, to exclude lateral gene transfer of proteins from another species, particularly bacterial. The proteins would have to contain mitochondrial targeting sequences to definitively distinquish them from prokaryotic homologs.