Appendix 1
Air flow in heat store while charging
(Or, How thermal stratification is preserved when the heat store
must accept air cooler than its hottest air)
David M. Delaney, June 12, 2006
 When
there is enough sun to make the column of air in the air heater hotter
and lighter than the column of air in the heat store, there will be a
flow of warm air from the air heater into the top of the heat store, and a
flow of cool air from the heat store into the bottom of the air
heater. There are no fans in the air heater or heat store, which operate entirely by natural (unforced) convection.
When
there is enough sun to make the column of air in the air heater hotter
and lighter than the column of air in the heat store, there will be a
flow of warm air from the air heater into the top of the heat store, and a
flow of cool air from the heat store into the bottom of the air
heater. There are no fans in the air heater or heat store, which operate entirely by natural (unforced) convection.
The design of the heat store is intended to
promote the formation, maintenance, and maximization of thermal
stratification, in which each part of the thermal mass is always at
least as warm as any lower part. In order to meet this goal, the
following statement must be true, or approximately true: Air
descends through the thermal mass of small stones only when, and
because, it is being cooled by the stones, or is at least not being
heated by them. Or, alternatively and equivalently: Air
descends through a part of the thermal mass of small stones only when,
and because, the descending air is at least as warm as the stones in
that part of the thermal mass.
If all air that flowed down through the heat store had to pass through
the thermal mass of small stones from its top to its bottom, these
statements would not be true, since convective forces could force
cooler air through a very hot top layer of stones, provided the stones
below were cooler than the entering air. The result would be to
decrease top-to-bottom temperature differences in the thermal mass, to
decrease thermal stratification.
The air space just north of the thermal mass has an important function
in preserving thermal stratification in the thermal
mass. It provides an easy flow path, much easier than the path through
the stones of the thermal mass. Air that is cooler than air at
a given height of the thermal mass may fall in this air space past the warmer part of the
thermal mass before entering it through the wire mesh that retains the north face of the thermal mass of small stones.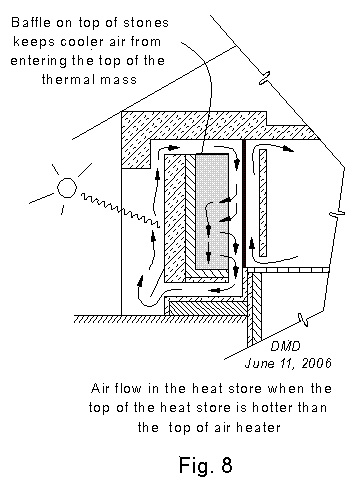 This bypassing flow
pattern preserves existing thermal stratification in the heat store
when air entering at the top of the heat store is not as hot as the
hottest air in the heat store. A baffle on top of the mass of
stones prevents air from entering it through its top. Air enters the
thermal mass only via the air space north of the thermal mass.
This bypassing flow
pattern preserves existing thermal stratification in the heat store
when air entering at the top of the heat store is not as hot as the
hottest air in the heat store. A baffle on top of the mass of
stones prevents air from entering it through its top. Air enters the
thermal mass only via the air space north of the thermal mass.
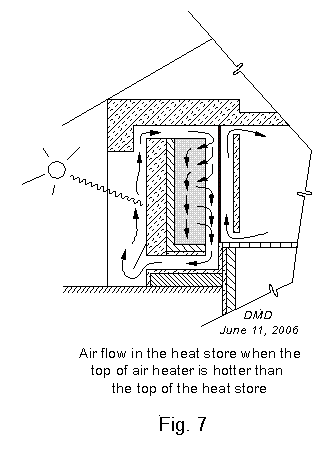
There are two general patterns for the flow of air within the heat store. In the first case, Fig. 7,
the air coming from the air heater is hotter than the air in the top of
the thermal mass of small stones. In the second case, Fig. 8,
the air coming from the air heater is cooler than the air in the top of
the thermal mass of small stones. The incoming air is always
warmer than the air in the lower part of the thermal mass of small
stones, of course, or there would be no flow into the heat store.
Return to main document