How do we quantify the absorption of UV-visible light?
When a beam of light hits a sample of the compound we are analyzing, some of it will be absorbed while some of it will pass straight through. The light that passes through - the transmitted light, can be measured. We know how much light is going in to the sample and can measure how much comes out so therefore we are able to calculate the percentage transmittance.
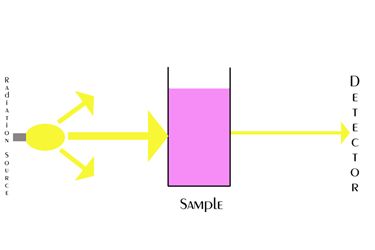
The diagram above is illustrating this concept with not as much light coming out as went in. It is however, more complex than this. The light/radiation is split into its constituent wavelengths before passing through the sample so we know what wavelengths are absorbed by the sample and how intense the absorption is.
By knowing the percentage transmittance we can calculate how much radiation is absorbed and this is what we are interested in.
Percentage Transmittance = (light out/light in) x100
The "light in" is known as the Incident radiation, I0 and the intensity of the transmitted radiation, "light out", is known as I.
If the percentage transmittance is 100% then it is obvious that no radiation is absorbed. However if the percentage transmittance is zero, then the absorbance value is infinite. This is the reason why the calculation of absorbance contains a logarithmic value.
A = log10 (I0/I)
This is an important equation as the A value is a component of the Beer-Lambert Law.
Example: If 50 units of radiation enters the sample and 20 units are transmitted through the sample: A = log10 (50/20) = 0.4

