What
happens when light enters a molecule?
As
we know radiation in the UV-visible region can promote an electron to a higher
energy orbital.
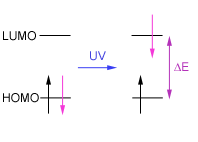
The
energy taken in or absorbed by a molecule is directly related to the amount
of energy (DE)
required to promote an electron from one orbital to a higher
energy orbital:
However,
UV-visible spectroscopy is particularly applicable to CONJUGATED
SYSTEMS e.g. b-carotene:

b-
Carotene is known as a chromophore. A chromophore
absorbs in the visible and UV regions, but if it absorbs in the visible region,
the molecule is seen to be coloured. Dyes usually have extended conjugated
systems as the conjugation causes the p-p*
transition (the transition between the filled and unfilled orbital) to be
reduced in energy. Visible radiation is lower in energy than UV radiation
and is capable of promoting an electron to the excited state, thereby conferring
colour.
Generally
the more conjugation in the molecule, the lower the energy required to promote
an electron. This is because the gap between filled and unfilled orbitals
is small and therefore not as much energy will be needed to promote an electron.





