Why do we use ultraviolet-visible radiation?
The wavelength of the radiation absorbed by a molecule corresponds to the energy required to promote an electron to a higher energy molecular orbital. Ultraviolet-visible radiation has the property of being able to cause these transitions of electrons between orbitals.
What are molecular orbitals?
When two atomic orbitals combine form a bond, 2 molecular orbitals are formed. For example, hydrogen, whose electronic configuration is 1s2:

The two 1s orbital are the hydrogen atomic orbitals. The electrons from these atomic orbitals come together in one of the molecular orbitals. This gives molecular hydrogen, H2. The electrons will go into the lowest energy molecular orbital - the bonding orbital. The unoccupied molecular orbital which is higher in energy than the atomic orbitals, is known as the antibonding orbital.
The number of molecular orbitals formed corresponds to the number of atomic orbitals we started with.
1,3-Butadiene
(C4H6) ![]()
1,3 butadiene has four p orbitals which are overlapping to give 2 double bonds:
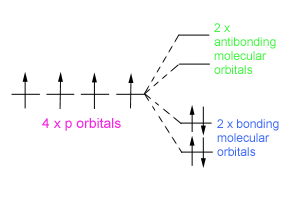
This diagram shows the molecule in the ground electronic state which means the electrons are in the lowest energy arrangement.
When this molecule is exposed to UV-visible radiation, an electron is promoted from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO).
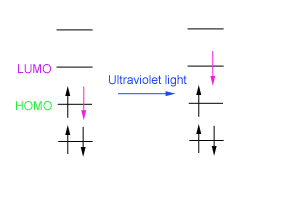
This transition is detected because when it occurs, radiation is absorbed. The UV spectrometer plots a graph of wavelength against absorbance so we can see at what wavelength absorbance occurs.
UV-visible spectroscopy is especially applicable to conjugated systems and this is discussed on the following page.

