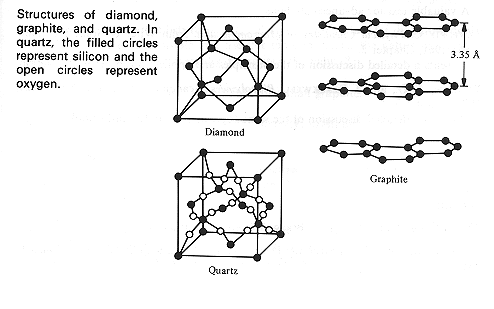
Covalent Crystals
![]() Introduction
Introduction
| Covalent crystals are formed when the atoms or molecules share their valence electrons equally. Covalent substances may have simple molecular structure or giant covalent structure. Giant covalent structures and covalent structure consists of millions of non-metal atom covalently bonded together in a structural network and there is no discrete molecule present. |
Giant Covalent Crystals
![]() Properties
Properties
1. High melting points and boiling points (strong covalent bonds have to be broken in order to melt or boil the substance) 2. hard and rigid(atoms are fixed in their position by strong covalent bonds) 3. poor conductors of heat and electricity (valence electrons of the substances are localized in covalent bonds, the electrons are not mobile) 4. insoluble in both polar and non-polar solvent |
¡@