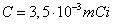|
|
Depleted Uranium Radiation
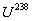 , or else known as
"depleted uranium", decays to , or else known as
"depleted uranium", decays to 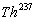 and emits an á particle of energy 4274keV(79%) or an á particle of energy 4151keV(20,9%) and a
ã photon of energy 49,55keV as the decay diagram shows.
and emits an á particle of energy 4274keV(79%) or an á particle of energy 4151keV(20,9%) and a
ã photon of energy 49,55keV as the decay diagram shows.

The disintegration constant is
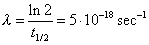 . If we consider a litre of . If we consider a litre of
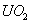 (which is produced by the impact
of a bullet (which is produced by the impact
of a bullet *) then the number of uranium atoms
is  . The activity of the
source is . The activity of the
source is 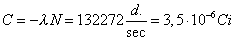 . .
The flux rate of the radiation at the distance
of one meter will be 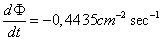 and at the
distance of one centimeter will be and at the
distance of one centimeter will be 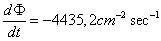 .
The exposure rate at the air is given by the expression .
The exposure rate at the air is given by the expression
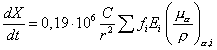 in units of in units of
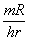 were the
activity is mesured in mCi, the distance in cm and the energy in MeV. In our case we have were the
activity is mesured in mCi, the distance in cm and the energy in MeV. In our case we have
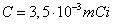
,  , ,
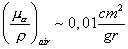 and so the exposure rate at the distance of one centimeter will
be
and so the exposure rate at the distance of one centimeter will
be 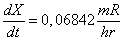 and at the distance of one meter will be and at the distance of one meter will be
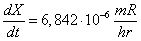 .
The dose rate is given by the expression .
The dose rate is given by the expression
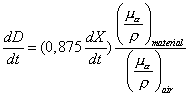 and if the exposure rate is in units of and if the exposure rate is in units of
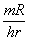 then the dose rate is in units of then the dose rate is in units of
 . .
In our case we will have:
|
distance
1cm |
time |
dose |
|
24h |
1,43mrad |
|
30d |
43mrad |
|
1y |
524mrad |
The equivalent dose is given by the expression
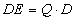 were Q is the
quality factor of the radiation, and if the dose is in units of rad then the
equivalent dose is in units of rem. were Q is the
quality factor of the radiation, and if the dose is in units of rad then the
equivalent dose is in units of rem.
In our case we will have:
|
distance |
time |
DE |
|
1cm |
24h |
1,43mrem |
|
30d |
43mrem |
|
1mm** |
1h |
5,98mrem |
|
24h |
143,69mrem |
|
30d |
4,31rem |
|
1y |
52,45rem |
Permitted limits
|
|
Professionals |
General population |
|
blood producing organs, bone marrow |
5rems/y |
0,5rems/y |
|
bones, skin |
30rems/y |
3rems/y |
|
limbs |
60rems/y |
6rems/y |
|
other organs |
15rems/y |
1,5rems/y |
If the one litre we have considered is placed inside
a lung and to all that is added the dose due to the á radiation and the dose of the
environment then things are becoming very worrying.
The above calculations are telling us:
- A 30mm bullet of depleted uranium stored in an ammunition warehouse doesn't
emit significant radiation and it can bee shielded without grate danger.
- When a bullet hits the armor of a tank, one part of it burns to produce uranium
dioxide and the rest of it remains in the air as dust particles. The dust particles are the most
dangerous because they are higher concentrations of uranium. If one inhales these particles or the
uranium dioxide, the quantity that will be absorbed by the organism will produce a significant radiation
dose which can be dangerous since the exposure will be for a long period of time an the source of
radiation will be very close to the tissue.
- If we try to detect the radiation that these particles add to the environment
we will probably fail due to the low concentration of these particles. So such measurements should not
be reassuring.
- Finally what needs to be pointed out is that the problem is created by the
accumulation of a significant quantity of uranium in the human organism. Because the organism doesn't
reject these substances, they accumulate to high concentrations. So it is not necessary the introduction
of the uranium to the organism to happen at once. We can have continuous introduction from the water,
the air and the food from the area of the bombing.
* Every
30mm bullet that was fired by the Á-10
aircrafts has 299gr of depleted uranium
that is capable of producing at list 1 litre of uranium dioxide(actually it is capable of producing
about 20 litres).
** I am interested in
that size scale because inside the organism these are the mean distances of the particles from the
tissue.
Here are some links on the subject of depleted uranium:
Extreme deformities in Iraqi children
Gulf War Syndrome Message Board
Where and how much depleted uranium has been fired?
DEPLETED URANIUM WEB ARCHIVE
PGU-14/B API Armor Piercing Incendiary [DU]
30mm Ammunition for the A-10 aircraft
M829 120mm, APFSDS-T(Armor Piercing, Fin Stabilized,
Discarding Sabot-Tracer)
And something on nuclear physics:
Glossary of Nuclear Terms
|
 . The activity of the
source is
. The activity of the
source is 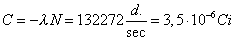 .
.
 ,
,
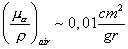 and so the exposure rate at the distance of one centimeter will
be
and so the exposure rate at the distance of one centimeter will
be 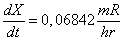 and at the distance of one meter will be
and at the distance of one meter will be
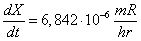 .
The dose rate is given by the expression
.
The dose rate is given by the expression
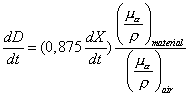 and if the exposure rate is in units of
and if the exposure rate is in units of
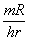 then the dose rate is in units of
then the dose rate is in units of
 .
.
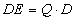 were Q is the
quality factor of the radiation, and if the dose is in units of rad then the
equivalent dose is in units of rem.
were Q is the
quality factor of the radiation, and if the dose is in units of rad then the
equivalent dose is in units of rem.

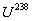 , or else known as
"depleted uranium", decays to
, or else known as
"depleted uranium", decays to 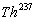 and emits an á particle of energy 4274keV(79%) or an á particle of energy 4151keV(20,9%) and a
ã photon of energy 49,55keV as the decay diagram shows.
and emits an á particle of energy 4274keV(79%) or an á particle of energy 4151keV(20,9%) and a
ã photon of energy 49,55keV as the decay diagram shows.
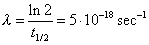 . If we consider a litre of
. If we consider a litre of
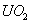 (which is produced by the impact
of a bullet
(which is produced by the impact
of a bullet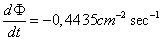 and at the
distance of one centimeter will be
and at the
distance of one centimeter will be 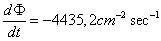 .
The exposure rate at the air is given by the expression
.
The exposure rate at the air is given by the expression
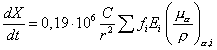 in units of
in units of