|
If we observe the wave model of an electron moving in the resonance orbit K of the atom of hydrogen, we verify that the particular arrangement of the wavefronts within the atom offers the opportunity for discovering several wave models which together justify in a deterministic way the energy emission. The difference between the image of the electron in resonance and non-resonance condition clearly explains the passage from one condition to another, and the causal reasons for photon emission in the intermediate conditions of existence, when it ranges from a resonance orbit with n = 2 to another resonance orbit with n = 1. In the internal photoelectric effect, a photon of right energy, which influences the atom, forces the electron to jump from the orbit K to the upper orbit L. There could be two wave causes with equal theoretical weight.
We can choose one of the two possibilities by asking a (perhaps new) question. How can the electron influence the atom? The electron in an atom moves in the resonance orbit and its motion can be the same or opposite to the incident photonís. Two distinct experimental cases could occur: one with the incident photon moving in the direction of the electronís motion, and the other one with the incident photon moving in the opposite direction. In the first case the electronís velocity should increase; in the second case there should be a decrease in velocity and therefore an energy loss which would force the electron attracted by the positive charge to fall toward the proton. Obviously, this case has never been observed; in fact if it were possible, matter at the atomic state would not exist.
We must consider that photon can never influence the atom in the direction opposite to the electronís rotation about the resonance orbit, and that the only presence of photonís waves in the resonance orbit causes the loss of the resonance condition. Probably both possibilities are partly true.
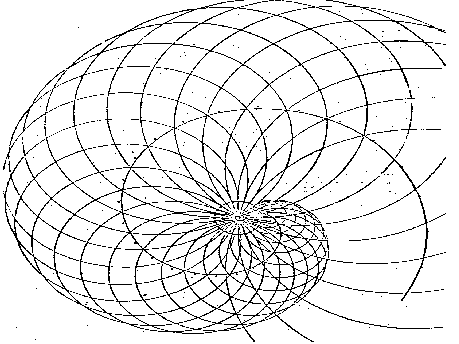
They could represent an invitation for the wave trains-photons which are able to enter the volute concavity of spiral, following the paths with minimum energy. And an obstacle for the wave trains-photons which, influencing the spiral convexity, are not able to follow those paths. A new orbital tube and a new resonance condition have appeared in the orbit L. The electron seems to be able to form a stable system in the new resonance orbit, which could remain indefinitely in motion about the proton without external interferences. But it is not true because the system is not stable. The system tends to return to the first resonance condition with n = 1 whatever the causes of unstability may be (a principle of minimum action, a consequence of resonance law, or another new law.) Firstly, let us examine its way of returning to the first resonance condition, considering the charge-body ratio, and without forgetting the wave nature of the phenomenon. What does it happen to a body in orbital rotation about a body of greater mass which attracts it, when the equilibrium which previously enables it to move in a stable orbit is upset? From our macroscopic experiences with artificial satellites, we know that a body is attracted by the gravitational field of a body of greater mass, and that it sets itself in decreasing elliptical orbits. As the body approaches the source of the attractive field, its velocity increases; when it moves away from the source, its velocity decreases, and it falls progressively toward the attractive body, reducing the radius of the elliptical orbits. As for the wave conditions of microworld where the dimensions involved are comparable to the wavelengths of interacting particles, the wave laws of diffraction come into play.The elliptical orbits of the wave train preceding the electron undergo a precession (in the same way as in the Mercury orbit). Perihelion rotates about the proton since the electronís wave train is forced to mantain temporarily the same distance from the center of proton due to diffraction. This happens every time the diffracted wave train interacts with the protonís waves at the shortest distance. The resulting orbits are less and less elliptical since their eccentricity gradually decreases as the electron approaches its fundamental orbit, while perihelion rotates about a decreasing spiral. During this series of orbits, the waves of the spherical involute-electron lose the characteristics of resonance condition, and have the same behavior as the waves emitted by any spherical wave source in motion along NON-RESONANT trajectories.
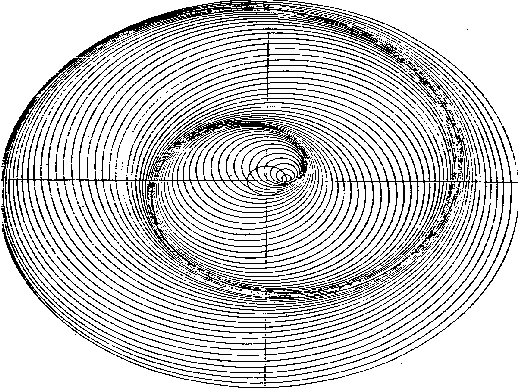
Fig. 30 shows geometrically the wave phenomenon deriving from the condition of circular motion of a wave source-particle in a nonresonant orbit. It seems logic to consider the compound wave generated by the motion of the electron about the elliptical orbits approaching the resonance orbit as the result of the frequency variation in a frequency modulation of the electronís elementary carrier wave. The light emitted from the atom shows that photon is not spherical, but it can be considered as a directional wave packet having a wavefront with defined surfaces; and this is justified by the hypothesis that the electron comes back following few elliptical orbits whose foci remain in the vicinity of a straight line during its passage from the upper resonance orbit to the lower one, or to the fundamental one. In such a case in fact only the portions of the wavefronts close to the straight line could be considered as parallel to one another. As a result, their propagation would be directional, while the positive energy of the wave packet would be the result of all the wave variations occured in the phenomenon. Then, the vave variables acting in the atom emission phenomenon only have a deterministic role. The photon that quantum mechanics considered as a secret quirk of fate, now has been revealed and shows all its causal wave mechanisms we have to understand. At this purpose, we have to change
our cause-effect relations so as to turn indeterminism from a destined
necessity into a mere experimental condition of incertainty derived from
our ignorance of the real and complete physical conditions producing the
cause. |