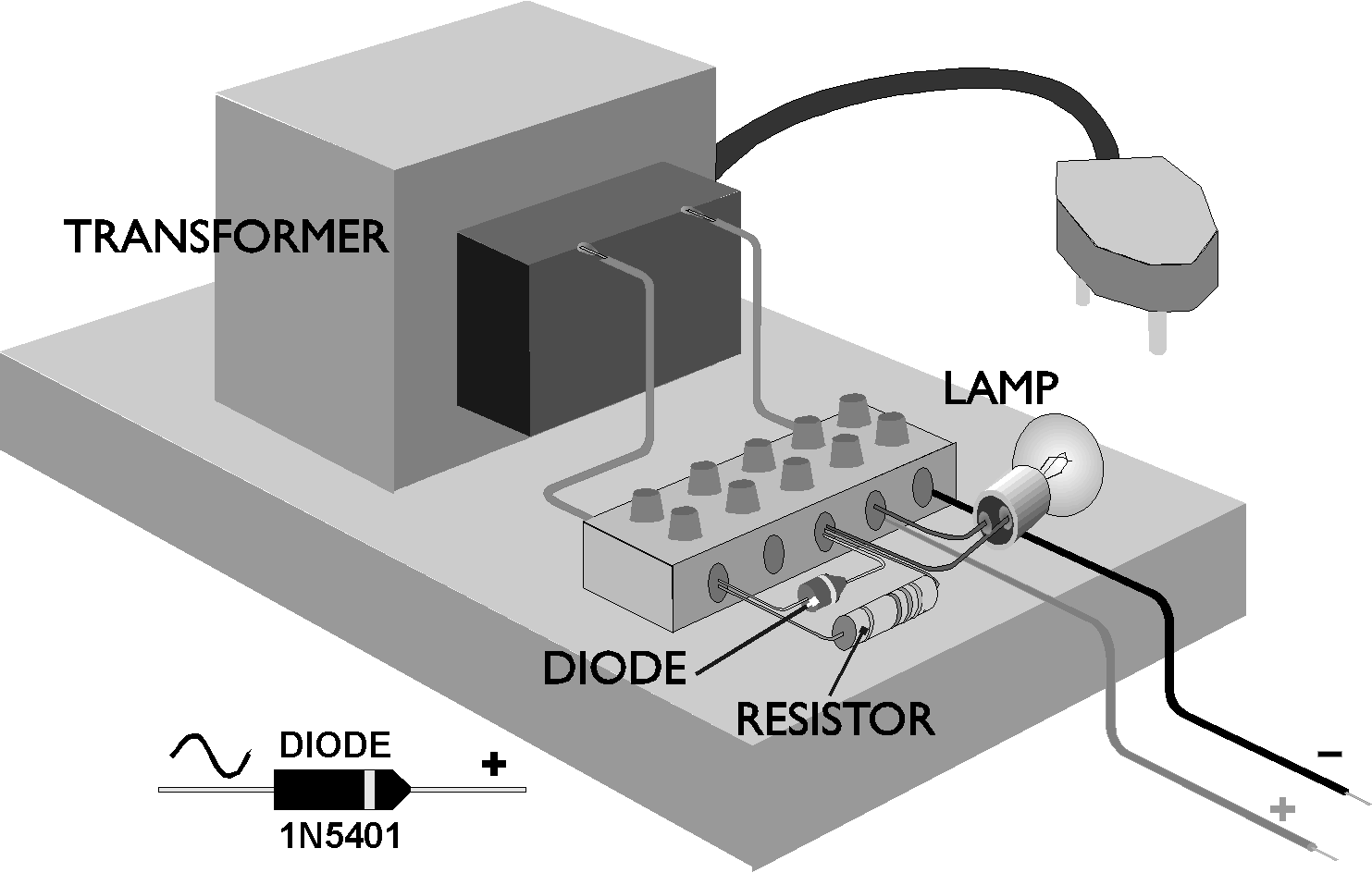
A Basic Nicad Charger
This article is primarily directed at those who use nicad batteries in their portable PCs and mobile phones. However, the information which follows is equally relevant to nicads used in camcorders and other domestic appliances.
Nickel Cadmium batteries are here to stay with us for a while yet so most of you will use them sooner or later. The nickel-cadmium cell was invented by the Germans during the last war. They were first produced by Deutsche Akkumulatoren Gesellschaft and were originally known as "Deacs" (from DeAk) in the same way as vacuum cleaners are still referred to as "Hoovers". (Note: One unit is a cell, two or more cells make up a battery.)
At the end of the war, the patent for the nickel-cadmium cell was offered as a part of German war reparations but the patent was rejected by the United States Patent Office because it was presumed to be much the same as the NiFe (nickel-iron) cell invented by Thomas Edison 50 years earlier. The NiFe cell was a poor second to the lead-acid cell as an accumulator because it would not hold a charge very well and would only generate 1.2 volts in comparison to the 2.0 volts of a lead-acid cell. However, a NiFe cell is virtually indestructible. It has steel plates and a steel case. If a cars were fitted with a NiFe batteries instead of lead-acid batteries, the battery manufacturers would soon be out of business. A NiFe battery could outlast several cars. Lead-acid cells have plates which are less robust than papier maché‚.
The original nickel cadmium cell had a big advantage over the conventional lead-acid and NiFe cells - it could be sealed because it didn't produce gas when being charged. (The sealed lead-acid cell was not invented then.) It also had the advantage that it could withstand a very heavy discharge rate without being damaged. This second factor has made model electric car racing possible; fully charged batteries are usually discharged within four minutes. Lead-acid batteries would be ruined by such treatment.
The sealed nickel-cadmium cell has three disadvantages when compared with a 2 volt lead-acid cell. A lead-acid cell voltage drops from 2.1 volts to 1.9 volts during 95% of its discharge cycle. A nicad generates only 1.25 volts on average, but its output voltage falls from 1.35 volts to 1.1 volts during discharge and it weighs more for the same ampere-hour capacity. On the other hand a nicad cell will last two or three times as long as a lead acid cell if it is treated correctly. (Some cells I use in my torch are over 20 years old.)
A modern NiCad cell is made like a double swiss roll inside. (Imagine chocolate and lemon sponge layers rolled together with cream in between.) Both 'plates' are made of steel. The cadmium plated cathode is an open net and the nickel plated anode is a fine woven mesh. The two plates are separated by a thin layer of porous plastic which acts like blotting paper. The separator is soaked in a potassium hydroxide solution. All nicads are fitted with a seal which acts as a safety valve to release gas generated within the cell. The amount of cadmium used is very small (it is just a coating) so the cell is not as unfriendly to the environment as some people believe. (Cadmium is widely used as an orange/yellow colouring agent for plastics and as a coating for screws.) The construction of a nicad cell should be borne in mind because it affects the response of the cell when it is misused.
Cadmium from the cathode is converted into cadmium hydroxide which goes into solution as the cell is discharged. The cadmium is plated back on the cathode when the cell is charged up again. It sounds simple but the charging/discharging process does not restore the cathode to its original state unless certain conditions are met. A slow discharge will remove cadmium from the cathode in a fairly even manner. A slow charge will cause cadmium to be more heavily plated on the parts of the cathode which are closest to the anode. This effect creates whiskers of cadmium which can grow during repeated charge cycles through the separator until they touch the anode. When whiskers have crossed the separator, the cell is shorted so that it cannot be charged any more. When charging stops, the whiskers are eroded a little by self discharging so that the short is removed but the cell ends up with a very much reduced capacity.
Both hydrogen and oxyen gases are generated through charging when a cell is already charged but they can recombine again to form water if the charge rate is low enough. This trick is achieved by having an anode which is much bigger than the cathode. If the cell is overcharged on a fast charger, the two gases will be generated more quickly than they can recombine. The safety valve releases the gas pressure but water is lost and the cell's capacity is reduced as a result. If the cell is overheated by very fast charging and discharging, steam can be generated and lost through the safety valve, and the plastic separator may melt. Very slow charging and discharging can cause the electrolyte to stratify so that its chemical activity is reduced. This gives the effect of reduced cell capacity. This problem also occurs if the cell is left unused for a long time. Stratification can be reduced and the cell's capacity restored by several cycles of fairly fast charging and discharging.
I have deliberately referred to cells so far because problems arise with batteries of several cells. No two cells in a battery are identical so some will have higher capacities than others. In a battery of cells, the battery will be effectively flat as soon as the weakest cell is discharged. This will leave most cells with some charge left in them. If the battery is further discharged, the weakest cell will be partially charged in reverse. Cadmium will be deposited on the anode and removed from the cathode. When the battery is charged again, the reverse charging of the weakest cell has to be overcome before proper charging can commence again. As a result the weakest cell is frequently undercharged and the process snowballs.
It shortens battery life and reduces total capacity. I don't know where the idea originated that nicads should be totally flattened. This notion has caused many otherwise salvageable batteries to be ruined. Whoever first published the idea that flattening NiCad batteries as a "good" thing should have all the ruined nicads heaped upon his head. I trust that this explanation of nicad battery operation will convince users that nicad batteries should never be discharged completely. Individual cells can be flattened but I fail to see any advantage in doing this anyway.
Nicads thrive on heavy use. They are ideal for electric screwdrivers and model cars. Careful fast charging and rapid discharging prevents stratification and reduces the rate of growth of whiskers. The ideal nicad charger is one which charges for 90% of the time and discharges for 10% of the charging time. The easiest way to do this is to employ a 'leaky' half wave rectifier in the charger unit. (Few commercial chargers employ this technique. If they did, the electricity suppliers would soon object.) On one half cycle the rectifier passes current normally and the battery is charged. On the other half cycle, the rectifier prevents current flow but a bypass resistor allows a small reverse current to flow and discharge the battery slightly. This charging method prevents the growth of whiskers altogether. Stratification can be avoided by charging the battery fast for the first 2/3 of the charge and charging normally for the final 1/3 of the charge. Nicads are made so that they can be charged indefinitely at the normal rate so it is possible to restore the charge in the weaker cells in a battery without damaging the cells that are already charged. The charging instructions on the side of every nicad indicate that the cell should be given a charge which is 40% more than the cell's capacity. Part of this is because of the inefficiency of the charge/discharge process and part is to help to prevent the weaker cells in a battery from being undercharged.
Some more advanced chargers include heat sensors. These detect when cells in a battery start to warm up. The conversion of electrical energy into a chemical change generates very little heat so charging cells stay cool. Charged cells have no more chemical to change so all the electrical energy is turned into heat and the cell gets warmer. A charged cell can accommodate the heat generated during normal charging but it rapidly deteriorates if it is overheated by fast charging for the reasons mentioned earlier.
Those of you who have the requisite electrical knowledge who would like to make up a whisker- preventing charger for their nicads can base the design on the following rules: (The discharge current should be 1/10 of the charge current.)
1. The transformer AC output voltage should be around 1.5 to
2 times the charged battery voltage.
2. Cells should be normally charged at 1/10 of their capacity
rating. e.g. A 2Ah cell should be charged at 200mA or 0.2A and
discharged (during charging) at 20mA or 0.02A
3. The rectifier diode should be rated at 5 to 10 times the normal
charge rate.
4. The series charging resistor value Rc is calculated as follows:
(V = volts, I = amps, W = watts).
Rc = (V transformer - V battery)/(I charge X 2) Wattage
W => I X R
(The current I charge is doubled because charging only takes place
during forward 1/2 cycles.)
5. The rectifier shunt discharge resistor Rd is calculated as
follows:
Rd = (V transformer + V battery)/(I discharge X 2) Wattage W <=
1W
6. These rules also apply to fast charging at 10 times the normal
charge rate. Fast charging should be carefully monitored and stopped
as soon as cell warming is detected.
As an example I will show how the values of the components are calculated.
Let us assume that you want to charge a set of four AA size
nicads at fast and standard rates.
Each cell will have up to 1.5 volts across it during charging
making 6 volts for the charging battery. Thre transformer output
AC voltage could therefore be 9 volts.
The normal charge rate for 500mAh AA cells is 50 mA. The fast
charge rate can be up to 500mA.
The charging voltage is effectively 9 volts - 6 volts = 3 volts.
The normal charge resistor would be 3/0.05 = 60ohms for full wave
charging but 30 ohms for the half wave charging required.
The fast charge resistor is therefore 1/10 of the normal charge
resistor and is 3 ohms.
The normal charge discharge current is 5mA and the fast charge
discharge current is 50mA.
The discharge voltage is 6 volts + 9 volts = 15 volts.
The normal discharge resistor is therefore 15/0.005 divided by
2 = 1500 ohms. The fast charge discharge resistor is 150 ohms.
The rectifier diode should be rated at up to 10 times the fastest
charge rate. A 1N5401 diode will suit. (It is quite common.)
The above example has a major shortcoming. A 1N5401 diode has around 0.5 volts across it when it is conducting. This reduces the charging voltage to 2.5 volts. The best solution is to start again but to use a 12 volt transformer instead of a 9 volt one. Now you know the sequence and the way the component values are calculated you can work out the values for any battery of cells and transformer rating.
A charger can be built using car bulbs as charging resistors. These have the advantage of showing how the charge is progressing and they can easily handle the heat dissipation problem. To calculate the resistance of a 12 volt car bulb use the formula: 144/wattage (V^2/W=R) A 6 watt tail light bulb = 144/6 = 24 ohms. A 21 watt stop light bulb = 144/21 = 6.8 ohms. Do not worry if the bulb resistances do not quite match the charging resistance required. It is best to have bulbs with voltage ratings as high as the transformer output voltage to prevent them burning out if the output is shorted. Two similar bulbs in parallel have half the resistance of one bulb. Two bulbs in series have twice the resistance of a single bulb and will withstand twice the voltage.

If a computer or video batttery pack seems to have lost capacity, charge it normally then discharge it with a car trafficator bulb. (Use a tail lamp bulb for mobile phone batteries.) Stop discharging as soon as the light output starts to dim. A good discharge rate is 10 times the normal charge rate. Repeat the charge/discharge process several times but let the battery cool off if it shows any signs of getting warm. Most battery packs are fitted with a safety fuse. If you short circuit a charged battery, the battery pack has had it unless you can repair a plastic container which has been broken open to replace the fuse.
A fully charged nicad cell will show just over 1.45 volts across its terminals while it is being charged at the normal rate. This voltage will fall to around 1.38 volts after being disconnected from a charger for half an hour. It will fall further to around 1.30 volts within a week without being used. A cell is effectively flat when the voltage has fallen to 1.15 volts. These voltages should be used to check if all the cells in a battery are working OK. A five cell battery should show around 6.9 volts when it is disconnected from the charger. If it shows 5.5 volts, one cell is dead. It is seldom possible to restore dead cells to normal working. A battery which contains one dead cell is effectively useless. If the cell is replaced, the new cell will still be working when another of the older cells has died.
If you need a new nicad battery, check with third party suppliers to get a better deal than the appliance manufacturer offers. The most popular size of cell used in bigger battery packs is the R size which is rated at 1.8 ampere hours. If you need a new battery pack, check to see if there is another make of battery which has the same voltage and ampere hour rating at a much lower price. If the shape of the pack is different, use the cells from the new pack to replace the cells in the original pack. Solder can be used to attach connections to the welded-on strips but never try to solder directly to the body of a cell. If the solder takes, the cell will have been ruined by overheating.
If the nicad pack in your portable computer will not last long enough for your needs, obtain a larger one of the same voltage and obtain a connector plug which is identical to the one on the original battery. Attach the connector plug to a suitable piece of twin cable and connect the cable to the additional battery. Make absolutely sure that the polarity is correct before plugging the connector into your computer. The external nicad will normally operate the computer in the same way as the internal battery or the mains power unit. The computer will now be a bit less portable but it will run it for hours without the battery going flat.
If you can use a soldering iron for electrical work, you should be competent enough to organise an additional battery supply. If you do not know the relevance of 60/40 solder, do not on any account play with your portable computer's power supply system. Get someone who is competent to do it for you.
 Thank you for visiting Wilf's Nicad Page.
Thank you for visiting Wilf's Nicad Page. 