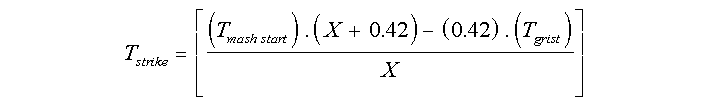PRE-MASH
SOME TERMINOLOGY !
Before the mash can get underway you must have your grain weighed out, the mash liquor heated to the correct temperature and, if you're that advanced, the pH of the mash liquor adjusted accordingly.
A question often arises though . . . if I want my mash to start at 68°C, how do I know what temperature to make the mash liquor prior to mashing in ?!
Well, first some terminology for you :-
- Any water used in the brewing process for tasks such as cleaning or rinsing is referred to by brewers as "WATER".
- Any water destined for uses that will see it end up actually in your beer is referred to as "LIQUOR".
- Before mashing you will need to have a source of hot liquor. The vessel you designate for this purpose is called the "HOT LIQUOR TANK".
- Your mash will be conducted in another vessel, called the MASH TUN.
- To start the mash you must first heat your hot liquor tank up to a pre-calculated temperature, call this T1.
- You then transfer a pre-calculated amount of the mash liquor (at temperature T1) across to your mash tun.
- Now, the mash tun is likely to be at room temperature and so adding in liquor of temperature T1 will see the mash tun warm up and the mash liquor subsequently cool down a little. After all, its just heated up the mash tun and so will end up slightly cooler than T1. You may think to ignore this . . . DON'T ! Its often a few degrees centigrade (e.g. 4-6°C) and this will be an error in your mash temperature . . . which you can't afford to leave up to thermodynamics, the fairies and other chance mechanisms.
- This initial temperature of the mash liquor in the mash tun, before the grain is added, is referred to as the STRIKE TEMPERATURE, call this Tstrike.
- Now, your grain will be sitting at a temperature also close to room temperature. This will depend on where its been stored all along and is worth measuring. Let's call it Tgrist.
- Being adept at brewing, you already know that the ratio of the liquor and the grain must be controlled, its a bit like making some breakfast cereal in the morning . . . too little milk and its rather stiff in the bowl, too much milk and it slops all over the place.
- For a single temperature infusion mash this figure would likely to be somewhere in the region of 2.3-2.8 litres of liqour per kg of grain. Call this X. Why not, its always good to have a variable X in a formula.
- In terms of some typical numbers, I may see my hot liquor tank heated to T1 = 82°C, with ambient temperature of 14°C. After transferring the hot liquor into the tun, it settles at Tstrike = 76°C.
- You've now got to imagine adding in grain at a temperature of say Tgrist = 16°C to mash liquor at 76°C and its anybody's guess as to where, somewhere in between, the mash temperature will eventually settle. This temperature will be your initial mash start temperature, let's call it Tmash start.
SOME FORMULAE !
Now, having been through all that terminology, there are two very useful formulae that you can use. The first allows you to calculate the amount of time it is likely to take in order to heat your hot liquor tank up from say 50°C where it is now to 82°C where you would like it to be. Try this, using temperature changes in Centigrade :-
The second formula allows you to calculate the strike temperature you need so that your mash starts off at the correct temperature :-
Knowing these makes life less of a guess ! Pop them into a spreadsheet and forever forget how horrible they look . . .
In fact, I've done it for you in my spreadsheet file, along with a heck of a lot more. Get the spreadsheet file !
The only other maths to consider is the total amount of liquor to transfer to your mash tun in the first place. For a single temperature infusion mash, go with 2.3 to 2.8 litres per kg of grain. Then, if you use a mash tun with a false bottom, add on the volume lost underneath it.
WHAT ABOUT THOSE MINERALS ?!
Ah, thought you'd ask that ! well, minerals are very important in brewing but most beginners either ignore them totally or go with the flow and add a teaspoon of gypsum into the mash liquor before mashing in the grain.
The reason the minerals are important are many fold but two of the most basic are :-
- Just as we sprinkle salt onto our food because it enhances the flavours in some undefined way, so adding mineral salts (i.e compounds similar in nature to table salt but containing different elements) to our mash liquor can alter our perception of the final beer's flavour components. Its subtle stuff and you often need to have many other factors sorted out in you process first before you'll notice the difference but heck, let's at least think about it !
- The presence of minerals in the mash liquor will affect the chemistry of the brewing process in ways far beyond your wildest dreams ! The most basic of these is that it helps control the relative acidity of your mash and this, in turn, controls to a large degree, the usefulness of the enzymes you're trusting to do the hard work. Not so subtle stuff and definitely worth thinking about !
The pH argument above is rather crucial in fact and the mineral profile of your water determines how the pH of the water changes once the grain is added. If the pH settles too high the mash can fail for one set of reasons, if the pH goes too low then it'll fail for other reasons. The efficiency of the mash can be badly affected and many other processes straight through to the fermentation and the beer's final stability can suffer.
Throughout history, differences in grain aside, brewers in different parts of the world showed an ability to make great lagers, porters or stout etc but often not in the same town. The reason was largely due to the mineral content of the water.
In places like Plzen in Czechoslovakia the water was VERY SOFT (i.e. low in minerals) and attempts to make pale lagers were very successful. In places like London (UK) the mineral profile saw great porters being made. The ales of Burton-on-Trent (UK), the German Dunkels, the Dublin stouts . . . the list goes on. However, it took mankind a while to wake up to this fact.
In your brewing you should first start with the mineral profile of your tap water, if there's a brewery in town it may be just fine ! If not, the local water authority can often help with an analysis. Explain you're a homebrewer, they'll be impressed !
A proper discussion of the desirable amounts for different styles is almost a book on its own but I will give you some info that I have been using for a while now. I rather like it because it goes beyond the usual arguments of how much calcium you have, or how much sulphate you have. It's secret, to me, is that it goes a step forward and considers the simultaneous effect of different amounts of the minerals.
In other words, it considers the effect of the ratio of each mineral to one another. This may sound less important than the overall amount but when two minerals work slightly against each other in their effect its quite important !
My source for this information, even though I have paraphrased, needs to be clearly stated and it is :-
"Brewing Liquor", a paper published by Dr. Keith Thomas in the September / October 1996 issue of "The GRIST International", a UK publication for small brewers, pages 27 to 29.
1. Look at your "total-hardness" figure in your water analysis as this gives you an indication of how much combined carbonate and sulphate salts are present.
Lower total-hardness figures are better for pale ales and lagers . . . say < 100 mg per litre (ppm).
Medium total-hardness figures are approximately 100 to 400 mg / litre and non pale beers are indicated.
High total-hardness are > 400 mg / litre.
2. Your alkalinity value tells you something about the amount of bicarbonates present. If its > 50 mg / litre then treatments to reduce it are indicated . . . check some of the books out in the suggested reading section for more on this. Miller gave a good account.
3. Calcium is one of the more important minerals in brewing, which is why simple instructions often suggest chucking a teaspoon in even if nothing is known about your brewing water. Better a little too much than none at all is the philosophy !
It has a major effect on the mash pH, more Ca gives a lower pH in the mash, yeast needs it for effective metabolism, it helps buffer the pH during sparging too. Check the level and make it 150 mg / litre almost irrespective of beer style . . . almost . . . maybe down it for those grists with high levels of roasted malts, you might undershoot your pH !
4. The sulphate level affects your perception of the beer's dryness and bitterness. Put in too much and it'll smell sulphury and we don't have to go any further on that one do we ! Too little and you'll miss something in those ales ! Go for levels of 250 mg / litre when making a bitter or other light beers.
5. Ever worried about your chloride levels ?! In beer, chlorides help mellow out the beer's flavour, softening it if you like. Levels > 200 mg / litre are indicated for beers such as milds, porters and strong, dark coloured beers. So if it mellows the flavour, you can probably guess that it and sulphate have been known to oppose each other in competing for your flavour buds !
6. The nitrate levels should be kept low as micro-organisms can get hold of them and make foul nitrosamines (what !?!?). If its below 50 mg / litre then you're not too badly off.
7. Sodium levels need to be kept under check as they can give salty or metallic flavours if present at > 100 mg / litre.
8. Potassium levels over 10 mg / litre can give salty or soapy flavours. Keep below this in your water source.
9. Magnesium can give a dry, astringent flavour if present at > 30 mg / lire.
OK, so what to do about the balances between them ?!
When making a bitter, for a dry, pale ale character, go for a ratio of 2 suplhate to 1 chloride; say 300 sulphate and 150 chloride. For a sweeter pale ale character go for 3 sulphates to 2 chlorides, say 225 sulphate and 150 chloride.
When making a mild or strong, dark beer, a sweet, full character can be obtained by balancing 2 sulphates to 3 chlorides, say 200 sulphate and 300 chloride.
When making a stout, keep the sulphate levels low and go for a chloride level of 200-400 mg /litre.
So, thanks to Dr. Thomas for this advice . . . its worth investigating !
So ends the Pre-Mash discussion !