PGHS-1 monomer with AA bound in the COX channel
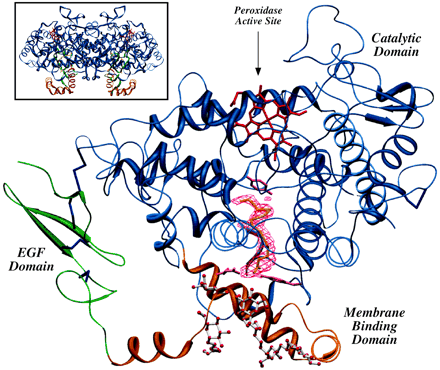
Fig.7 A ribbon representation of the Co3+-oPGHS-1 monomer with AA bound in the COX channel. The EGF domain, MBD, and catalytic domain are shown in green, orange, and blue, respectively; Co3+-protoporphyrin IX is depicted in red, disulfide bonds (Cys36-Cys47, Cys37-Cys159, Cys41-Cys57, Cys59-Cys69, and Cys569-Cys575) in dark blue, and side chain atoms for COX channel residues Arg120, Tyr355, and Tyr385 in magenta. (7)
Fig.8 AA bound in the COX channel. (A) Stereo view of AA (yellow) bound in the COX channel. Side chain atoms for all residues that contact the substrate at the carboxylate, C-2 through C-11, and C-14 through C-20 are colored red, orange, and green, respectively. Ser530 (magenta), which is acetylated by aspirin, lies below Tyr385 (gray), the likely radical donor during catalysis. Leu531 (light blue) lies above Arg120 but does not contact the substrate. The two red spheres represent the presumed location of O2 for attack on C-11. (B) The view of the COX active site rotated 90° about the vertical axis [using the same color scheme as in (A)]. Residues Phe518, Leu384, and Met522 (in light blue) along with Phe381 and Trp387 constitute the endoperoxide pocket. (7)