AVIATION
GASOLINE BASE STOCK FROM CONVERSION PROCESS:
Many conversion process have been develop for the production of high antiknock aviation gasoline. The greater percentage of the regular and premium grades of this type of fuel consists of conversion products.
The following general classes of conversion processes have been developed.
TYPES OF CRACKING:
There are two types of cracking thermal and catalytic cracking.
In this process, the fuel which may be gas oil fuel oil (heavy or light) atmospheric or vacuum residue is heated upto 450 – 750 oC at pressure ranging from 1 – 70 atm to produce gas gasoline etc. Octane number of gasoline obtained may be up to 75. The yield and quality of the gasoline obtained will depened upon the type of feed temperature and pressure.
Feed residue of vacuum distillation of crude yields gasoline = 55% , Diesel = 20% , Fuel oil = 15%, Coke = 10% , Octane number of gasoline = 92.
(b) Polymerization:
The tem polymerization may be define as combination of smaller hydrocarbon molecules to form largers molecules. This method utilizes the waste gases from cracking processes to form hydrocarbons boiling in the gasoline range. This increase gasoline yield and also quality since the polymer gasoline has a relatively higher octane number.
Only unsaturated compounds will combine under polymerization conditions and the product is generally olefin in nature. Polymerization is carried out under elevated temperatures and pressures. High temperatures in general tend to crack rather than polymerize low temperature and higher pressure favour polymerization. Catalysts are used under modified temperature and pressure conditions to increase the yield.
(c) Reforming:
Reforming means arrangement of molecules with out much affecting the average molecular weight of feed which is generally naphtha of gasoline boiling range. Reforming is carried out to produce high quality gasoline by heating, with or with out catalyst, the naphtha.
Reforming can be thermal or catalytic as in the case of cracking. Catalyst a part from accelerating the process also enhance the yield and quality of gasoline. The gasoline produced by reforming is called reform gasoline or reformat. The reform gasoline has a wider boiling range than the feed and its volatility is increase.
Octane number increases in the order paraffin ® olefin ® naphthane ® isoparaffin ® aeromatic.
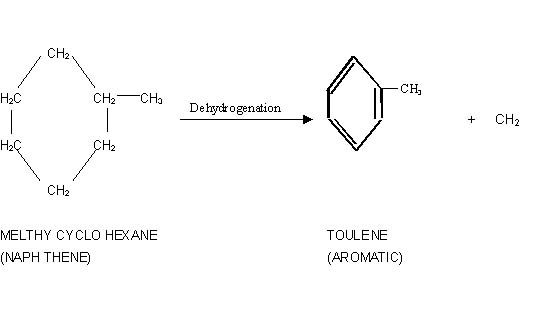
The main reaction in the reforming process is dehydrogenation
of naphthene to produce aeromatics. Other reactions occurring during reforming
are dehydrocylization of paraffins isomerization, hydrocracking, dehydroisomerization,
dehydrogenation etc.
1) Dehydrogenation of naphthene
to aromatic.

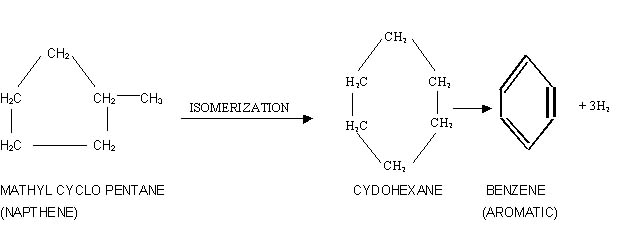
2. Dehydroisomerization:
In a typical reaction methyl eyclopentane isomerises
to cyclohexane which dehydrogenates to yield benzene.
3. Dehydrocyclisation of Paraffins to Aromatic.

4. Hydrocracking of higher paraffins to lower paraffin’s.

Decane n – pentane Iso – pentane
Lower boiling point paraffins have higher octane number.
5. Isomerisation of paraffins:
Here , n – paraffins are converted into iso paraffin
which have higher octane number.
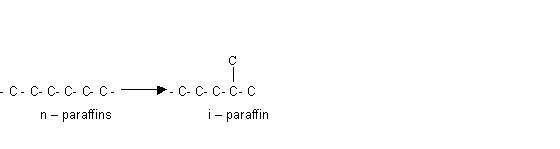
though olefins have higher octane number than corresponding n-parffins still this reaction is undesirable b/c lower olefins polymerize to produce gummy materials which is un wanted.
×
Ø
|Chemical|
Faisalmurad|Guest book|
Comments|WEBMASTER|D.C.E.T|N.E.D uet|Title|
Certificates|MAIL Us|