
WATER as FUEL - WATERFUEL - WATERCAR - WATERGAS - WATERBURNER - WATER FIRE - WATERPOWER - WATERBREAKER - WATERERA - WATERMOTOR - WATERENERGY -
---------------------------------------------
Home -Introduction -Conclusion -Site Map - Links
Water Electrolysis Systems - Water Explosion Systems - Water Plasma Systems - Other Water Dissociation Systems - Other Systems
---------------------------------------------
Electrolysis menu : CARS - LORRIES/GENERATORS - WELDING - HYSTORY and PATENTS - TRICKS - OWN REALIZATION
#>#># Click here for a LARGER and UPDATED version of this website #<#<#
REVIEW of TRICKS to MAKE an EFFICIENT an SAFE ELECTROLYZER :
# - About the PLATES for the WATER ELECTROLYZER :



Giving SAND PAPER or MACHINING the plates surface to increase the gas production :

--------------------------------------------- Return to the top
# - About SECURITY for an ELECTROLYZER :

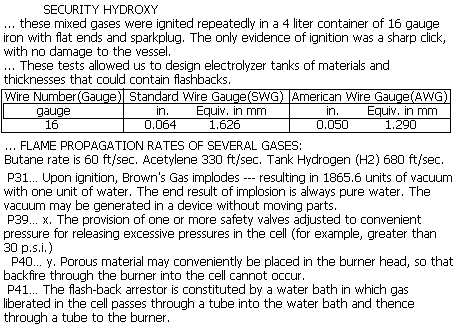
--------------------------------------------- Return to the top
# - About the ELECROLYTE to use in the system :
- The water : Tap water can be so unreliable because of the dissolved solids.
Personally I use ONLY distilled/dionized water (water for battery without the acid) + Sodium Hydroxide (Lye/Red devil drainer).
- The Electolyte : Used properly, with 304 or 316 stainless steel, NaOH or KOH make a very clean and efficient catalyst. Be careful of these chemicals because they can burn your skin and eat nice little holes in your clothes.
- NaOH : LYE, sodium hydroxide, drain opener powder, caustic soda
1...Lye is made of sodium, hydrogen, and oxygen, the only products of reaction can be hydrogen and oxygen, and the only byproduct is a very tiny bit of sodium precipitation over the long haul = NO TOXICS CREATED
2...For best electrode life lye and distilled water is the way to go. Good quality 304 or 316 works well, 316 is best, the electrolyte or the electrodes do not get consumed, just the water. While many other materials will work, the unwanted reactions consume material along with the water and create unwanted byproducts. only need to add distilled water to the electrolyzer, while Lye stays in the electrolyser.
3...Lye dilution rate : electrolyte concentration : Start with 1 teaspoon (for SERIES-CELL design) of the Red Devil lye & see what it does. If it's not enough you could add more…, then let it run and heat up, checking current draw. If needed, add more and wait for the heating to stabilize, until you reach your target operating current.
4...Red Devil; Lye Crystals. Just be sure it is at least 99.99% pure Do NOT use any electrolyte but pure sodium hydroxide (NaOH). There are many reasons, including efficiency, materials compatibility, foaming, etc.
5...NaOH NOT COMPATIBLE with PVC = Polyvinyl Chloride see http://www.eskimo.com/~ghawk/h-o/chemchrt.htm
6...NaOH is compatible with this plastics : LDPE = Low Density Polyethylene // HDPE = High Density Polyethylene // PP = Polypropylene // PA = Polyallomer // PC = Polycarbonate, Lexan // PSF = Polysulfone // PMP = Polymethylpentene // FEP - PFA = Teflon // ECTFE/ETFE = Halar/Tefzel // PS = Polystyrene // PVDF = Polyvinylidene Fluoride.
7...The NaOH solution saturated my resin containers in numerous places and eventually leaked through. Lye is used as a filler in some fiberglass resin hardeners. it's working beautifully as a connection cement. Lye is not used in all fiberglass resin hardeners.
8...(NaOH). Sodium hydroxide is one of the most commonly used industrial chemicals, used as a strong-base cleaning agent or to neutralize acidic processes. This is used in everything from soaps to plastics, paints to drain cleaners. Your dentist even uses it to clean your teeth before filling.
9...Sodium hydroxide has a reactive affinity with aluminum and thus can corrode any aluminum parts like heads or pistons if it gets into the fuel line. This, however, is prevented by any simple bubbler, connected along the fuel line and by the design of the reactor chamber and gas storage buffer system. In a properly designed system, NO NaOH will get into your engine.
- KOH : Potassium Hydroxide,
1...KOH is more expensive but better performance than NaOH. Maintain as close as to 28%KOH.
2...I am using 304ss and KOH in plastic containers (rubbermaid or Glad brands) and my plates and electrolyte consistantly turn brown within days with brown particulate (I assume to be rust) settling to the bottom when the cell is turned off
3...KOH NOT COMPATIBLE with PC = Polycarbonate, Lexan - see http://www.eskimo.com/~ghawk/h-o/chemchrt.htm
4...KOH is compatible with this plastics : LDPE = Low Density Polyethylene // HDPE = High Density Polyethylene // PP = Polypropylene // PA = Polyallomer // PVC = Polyvinyl Chloride // PSF = Polysulfone // PMP = Polymethylpentene // FEP - PFA = Teflon // ECTFE/ETFE = Halar/Tefzel // PS = Polystyrene // PVDF = Polyvinylidene Fluoride
- Look on the bottom of bottle, if recycling triangle has No 2 or 5, bottle is chemical resistant for KOH and NaOH. -
NaOH and KOH will attack aluminum, that's why we use bubblers to dry and filter the vapors of electrolyte.
- Baking Soda, bicarbonate : I suggest you avoid baking soda, or salt for that matter. You cannot see, taste, or smell, the nearly 30% carbon monoxide that using baking soda in electrolysis generates. At least you can smell the chlorine from using salt. Mon Nov 14, 2005 oupower.com. Baking soda is consumed by the reaction. Unless you're wanting to kill yourself and any loved ones.
- About using SALT (standard table/sea salt) :
- 1...In one of these threads Bob wrote that using salt produces chlorine gas. It puts out large amounts of deadly chlorine gas, which becomes a health hazard for anyone in or near the vehicle.
- 2...On top of that, chlorine is highly corrosive, much more so than salt by itself. It will rapidly take its toll on the engine components. It will also destroy the electrode metals, even stainless steel is vulnerable to it.
Salt is one of the worst things to use as an electrolyte, it produce deadly gas, and eat up the plates in very short time!
(Cheaper still is old white distilled vinegar (not tested yet about toxicity and plate corrosion))
--------------------------------------------- Return to the top
#>#># Click here for a LARGER and UPDATED version of this website #<#<#
# - about the problem of 'water freezing' in cold areas :
- Insulation doesn't prevent the transfer of heat, it slows it. Therefore the heat in the unit would be exchanged slower, and it would take longer to freeze.
- "A solution of 20 parts calcium chloride in 80 parts of water congeals only at 5 degrees F above zero."
- Both ethylene glycol and propylene glycol are clear, colorless, slightly syrupy liquids at room temperature.
- Alcohol evaporates faster than water in solution so any excess heat (inefficiency in electrolysis) the alcohol vaporizes and is sucked into the air intake and the water is left behind and if the temperature gets too high the ethanol turns into methanol which is deadly. A few drops can kill you if swallowed.


--------------------------------------------- Return to the top
#>#># Click here for a LARGER and UPDATED version of this website #<#<#
# - More about ELECTROLYSIS of WATER:





--------------------------------------------- Return to the top
# - About electronic for HYDROXY :
with 6 cells in series, from 12-14V from a car, each cell will get around 2 volts, what is closed to the minimum needed for electrolysis to happen, so the efficiency will be very high, the heat no to important, and then no need for electronic, only a relay to start/stop the system with the engine, that's my conclusion after months of practical testing of many kind of design.
Cheaper, more Reliable because no electronic, and also very efficient and can be made by any iron engineering work shop only.
Electronic is usually here to reduce the voltage on the electrodes, to reduce the heat in the cell for the water not to reach boiling point, but it heat up the MOSFET, so it needs also an huge and expensive heat sink not to burn out the transistors;
and electronic is used also to resonate the electrodes ( the capacitor formed by the electrodes) but it is first very difficult to obtain this resonance, and secondly the frequency of resonance will always change, being influenced by the water temperature, that rise with time in the cell, and by the water conductivity, that change with the gasification / evaporation of the water, making the catalyst more concentrated in the solution; so it's difficult to keep this frequency / pulsing at resonant level …
This 'resonant frequency' of the electrodes / capacitor call the intervention of Radiant Energy, that is when you send a pulse to the capacitor, a more important current is induced in it when the pulse stop brutally. And this Cold Electricity 'current' being used also in the electrolysis process of the cell, you get more gas produced with the same power send, just by using this DC pulses that will initiate the coming of the Vacuum Power to help in your job. Read about Tesla, Bedini, Bearden and many others, in the field of Zero Point Energy (its most famous name) and you will understand how Meyer's cell worked, giving a lot of gas with almost no electric power used…
It seems that Utopia Technology succeed in this, by using sensor sand a processor to always adjust the right frequency, following the evolution of the parameters. But they won't tell you that they use the Vacuum Energy Excitation to get it to involve in their electrolyser's work.
The second concept involving 'resonant frequency' is the one that will resonate the water molecules and not the electrodes; it's a frequency that increase the energy inside the water molecule until to much of it bring the molecule to split, the atoms to separate ... read about Keely discovery of resonant frequency to split water, 100 years ago, or see the recent Mateiro video and you will understand that if you find a frequency to split water directly, it will have huge effects that seems difficult to control with a simple apparatus (like INSTANTLY converting any volume of water in its gases, 1.800 times more voluminous…). This is not done by any know actual company/product…
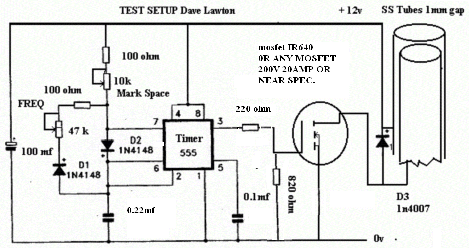


--------------------------------------------- Return to the top
#>#># Click here for a LARGER and UPDATED version of this website #<#<#
# - Pulsed DC electrolysis :



--------------------------------------------- Return to the top
# - About CAR TIMING adjustment :



--------------------------------------------- Return to the top
#>#># Click here for a LARGER and UPDATED version of this website #<#<#
# - About Internal Combustion Engines :



--------------------------------------------- Return to the top
Electrolysis menu : CARS - LORRIES/GENERATORS - WELDING - HYSTORY and PATENTS - TRICKS - OWN REALIZATION
---------------------------------------------
Home -Introduction -Conclusion -Site Map - Links
Water Electrolysis Systems - Water Explosion Systems - Water Plasma Systems - Other Water Dissociation Systems - Other Systems